Carbonate Alkalinity As Caco3
This explains why its so much more difficult to keep calcium in solution over 83 pH. During the experiment the water is taken in a conical flex and the acid is taken in the burette.

Solved Calculate Carbonate And Bicarbonate Alkalinity Of Chegg Com
P Alkalinity is the alkalinity that is determined by using phenolphthalein indicator.

Carbonate alkalinity as caco3. CaCO 3 s Ca 2 CO 3 2-CO 3 2- H 2 O HCO 3-OH-. To calculate alkalinity as calcium carbonate use this formula to calculate the alkalinity in concentration of calcium carbonate CaCO3. The best pH range for aquatic life to function properly is 60-90 pH.
Calcium Carbonate ion Calcium Carbonate. The carbonate alkalinity refers to the sum of the concentrations in solution of the free HCO3- and CO3-- species. So it is not possible to prepare a solution with 1 gl but instead you can use sodium carbonate the soloubility 217 gl.
Alkalinity prevents sudden changes in the. Carbonate Alkalinity as CO32- mgL 06 Carbonate Alkalinity as CaCO3 mgL 06 150 90 mgL. If your lab results list the bicarbonate and carbonate contributions to carbonate alkalinity separately and both of them are mgl as CaCO3 just add them together.
When CaCO 3 dissolves in water the carbonate CO 3 2- can react with water to form bicarbonate HCO 3- which produces hydroxide OH-. The endpoint volume alkalinity and alkalinity as calcium carbonate for each sample are displayed in table 2. And Pitt 2002 in.
What does high alkalinity in water mean. -Alkalinity as mgL CaCO3. The alkalinities for the samples were calculated using Equation 3.
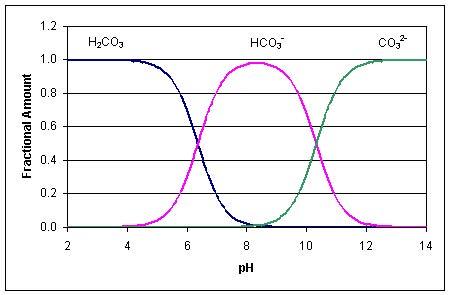
Alkalinity is an important parameter because it can directly affect aquatic life. When a waters pH is above 83 its alkalinity tends to come from carbonate ions and below that threshold the alkalinity usually comes from bicarbonate ions. Show activity on this post.
Alkalinity is usually measured in milligrams per liter of calcium carbonate which is a calcium ion bound to a carbonate ion. Dear all I am plotting multiple data points in Gtplot 70 and one of the plots I use is a Piper plot which has the variable HCO3 CO3. Insert the values into the formula to determine the alkalinity as CaCO3.
What we need is the equation for the material balance of the system. As a result the carbonate fraction of hardness expressed as CaCO 3 equivalents is chemically equivalent to the bicarbonates of alkalinity present in water Burton Jr. In this case 2P is more than T so we read the fourth row of the table.
Alkalinity can increase the pH make water more basic when the alkalinity comes from a mineral source such as calcium carbonate CaCO 3. I just read that I need to have a column for carbonate alkalinity as CaCO3 in units of mgkg in the scatter data file. Alkalinity can increase the pH make water more basic when the alkalinity comes from a mineral source such as calcium carbonate CaCO3.
Carbonate Alkalinity as CO32- mgL 06 Carbonate Alkalinity as CaCO3 mgL 06 150 90 mgL. When CaCO3 dissolves in water the carbonate CO3 2- can react with water to form bicarbonate HCO3- which produces hydroxide OH-. 2P - T 250 - 80 20 ppm.
Alkalinity is waters capacity to resist acidic changes in pH essentially alkalinity is waters ability to neutralize acid. Carbonate alkalinity is especially important in environmental contexts. These numbers depend on the assumption that the methyl orange end point is 43.
The alkalinity as calcium carbonate was found using the molecular weight of calcium carbonate as described in Equation 4. Total alkalinity 150 mgL as calcium carbonate contains. Carbonate Alkalinity as CO 3 2-mgL 06 Carbonate Alkalinity as CaCO 3 mgL.
I then converted 125 meqL to eqL. The result can be expressed as mgl CaCO3. Thus P Phenolphthalein alkalinity as CaCO 3 in mgL T Total alkalinity as CaCO 3 in mgL Controversy 1.
Associated with the bicarbonate and carbonate fraction of alkalinity are the principal cations responsible for hardness usually Ca and Mg Equations 3 and 4. As a result our water contains 80 ppm bicarbonate alkalinity and 0 ppm hydroxide and carbonate alkalinity. Lets assume that P50 ppm and T80 ppm.
Confusion exists with CaCO 3 as it does not always have an equivalent weight of 50 mgmeq Snoeynik and Jenkins 1980 Water Chemistry when converting eqm3 to mgL 2. Carbonate ions are very attracted to calcium so they create calcium carbonate and precipitate out of solution. The problem is the soloubility of calcium carbonate is very poor.
Answer 6250 mgl CaCO3-Approximate the total carbonate concentration CT in moles per liter. A water body with a high level of alkalinity which is different than an alkaline water body has higher levels of calcium carbonate CaCO3 which can decrease the waters acidity. It is 14 mgl.
Cs ceCaCO3 ceH2CO3 HCO3- CO32- Where Cs here stands for the known concentration of the salt calcium carbonate. Reason is that occasionally CaCO 3 has a valence of 1 instead of 2 giving it a mgmeq. To get the same molarity you have to dissolve 106 g of it.
As we assumed all carbonate came from calcium carbonate we can write. Ct for such a sample is 20 and the P alkalinity would be 52 ppm as CaCO3. Total alkalinity 150 mgL as calcium carbonate contains 90 mgL alkaliniy as carbonate ion.
Carbonate species contribute to the alkalinity than other basic species does because considerable amounts of carbonate species are found in water naturally. Using the approximation formulas the carbonate calculates as 63 mgL and the bicarbonate as 1287. This alkalinity is easily measured by titration with a standard solution of N50 sulfuric acid.
Answer 0125 moles per liter Homework Equations Not certain The Attempt at a Solution I was able to determine the alkalinity by finding the equivalent weight of CaCO3 50 geq. Ca CO3-- CaCO3. Alkalinity of natural water is mainly due to the presence of two forms of the carbonate ions denoted as HCO3- and CO32- that act as a buffer system.
Alkalinity buffers water against pH changes. Calculate alkalinity in terms of calcium carbonate using titrate volume titrate concentration water sample volume a correction factor based on the titration method and the conversion factor for milliequivalents to milligrams of calcium carbonate. To specify this value click analyte - Chemical parameters - Carbonate alkalinity.
CaCO3 s Ca 2 CO 3 2-CO3. Carbonate for buffering just like CaCO3. Converting Carbonate Alkalinity from mgL as CaCO 3 to mgL as CO 3 2-CaCO 3 has a molecular weight of 100 gmol The CO 3 2-anion has a molecular weight of 60 gmol Therefore each milligram of CaCO 3 contains 60100 06 mg of CO 3 2-The conversion is as follows.
We can calculate the components of the alkalinity as follows.

Ppt The Geochemistry Of Natural Waters Powerpoint Presentation Free Download Id 1994642

Pdf Electrochemical Splitting Of Calcium Carbonate To Increase Solution Alkalinity Implications For Mitigation Of Carbon Dioxide And Ocean Acidity Semantic Scholar
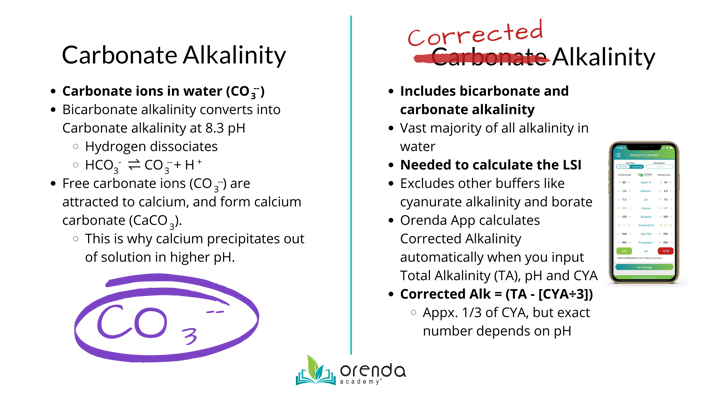
Carbonate Alkalinity Vs Corrected Alkalinity
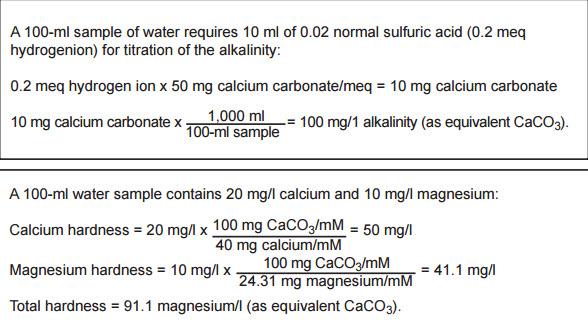
Total Alkalinity Total Hardness

Carbonate Alkalinity Vs Corrected Alkalinity

Values Of D 13 C Caco3 D 13 C Dic And Alkalinity Of Analyzed Rock And Download Scientific Diagram
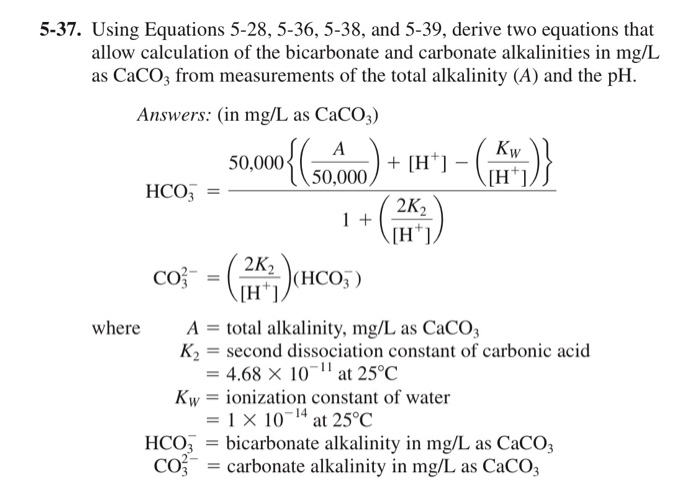
Solved 5 37 Using Equations 5 28 5 36 5 38 And 5 39 Chegg Com
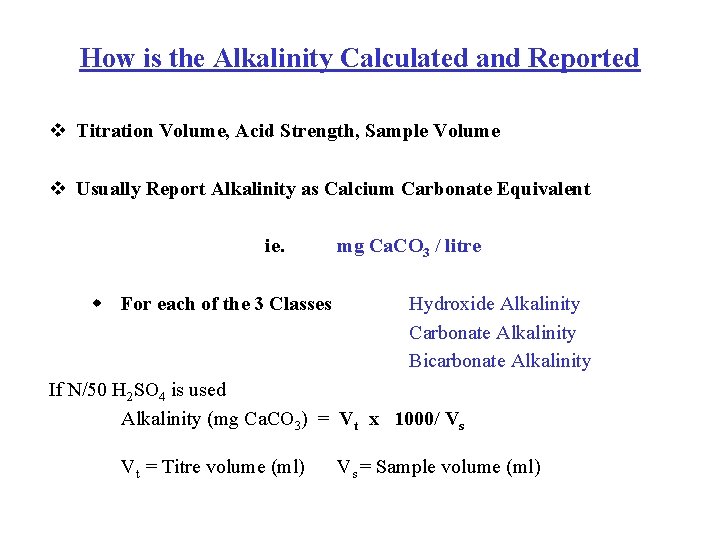
Acidity And Alkalinity Definitions Alkalinity Measurement Task Acidity

Predicting Calcium Carbonate Scaling Accurately 2019 01 11 Process Heating
Carbonate Alkalinity Mg Caco3 L 0 820 Hco3 Chegg Com

Solved Calculate The Bicarbonate And Carbonate Chegg Com

Pdf Electrochemical Splitting Of Calcium Carbonate To Increase Solution Alkalinity Implications For Mitigation Of Carbon Dioxide And Ocean Acidity Semantic Scholar

Posting Komentar untuk "Carbonate Alkalinity As Caco3"