[Tanpa judul]
Baking soda is a mixture of sodium hydrogen carbonate and tartaric acid. CaCO 3 CaO CO 2.
What Will Be Produced If Acid Meets Calcium Carbonate Quora
Add 125 cm 3 10 M hydrochloric acid to the flask and click the pause button on the main toolbar to start the simulation.

Carbonate + acid →. The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid. In this experiment a solution of Na2CO3 will be titrated with a solution of HCl. As it involves the release of carbon dioxide a brisk effervescence is seen.
Reactions involving calcium carbonate. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. This is so you can start all of the experiments at the same time so they are a fair test.
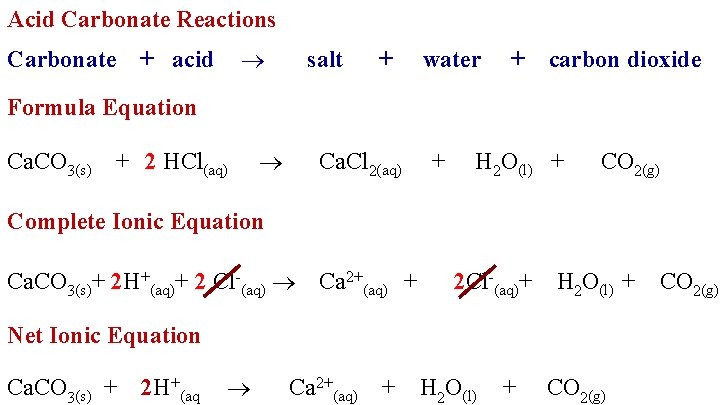
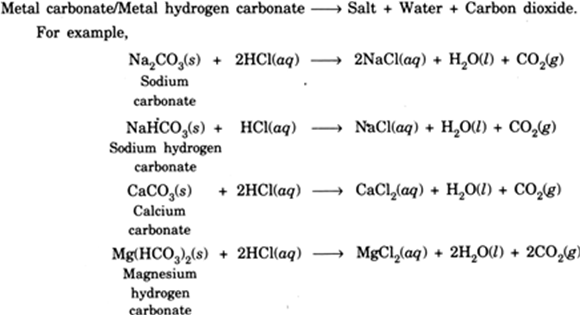
Also HCl can be added to solid Na 2 CO 3. Calcium carbonate and hydrochloric acid reaction is an exothermic reaction. The reaction between an acid and a carbonate can be represented by the general word equation shown below.
Carbonates are readily decomposed by acids. When it reacts with dilute acid it liberates carbon dioxide as a by-product. At 1200K calcium carbonate decomposes to give carbon dioxide and calcium oxide.
Carbonic acid can be considered to be a diprotic acid from which two series of salts can be formednamely hydrogen carbonates containing HCO 3. Titration of Sodium Carbonate with Hydrochloric Acid. It is safer to handle thats why it is preferred over sodium hydroxide in various chemical processes.
Decomposes at high temperature 825C to give gaseous carbon dioxide and calcium oxide quicklime. Part 1A Prepare a 02 M HCl solution from 6M HCl Pour 250 mL of ultrapure water into the 500 mL glass bottle Pour 16667 of 6M HCl into the glass bottle Cap the glass bottle and shake for 1 min. Solvay process is used for the production of sodium bicarbonate and sodium carbonate industrially.
Carbonic acid H 2 CO 3 is formed in small amounts when its anhydride carbon dioxide CO 2 dissolves in water. The name of the salt is determined by the metal part of the carbonate and the acid used. When aqueous hydrochloric acid is added to aqueous sodium carbonate Na 2 CO 3 solution carbon dioxide CO 2 gas sodium chloride NaCl ad water are given as products.
CALCIUM CARBONATE is non-combustible. Sodium carbonate is one of the common compounds we used in our home for purposes like washing clothes also known as washing soda or soda ash. Acid carbonate salt CO 2 water.
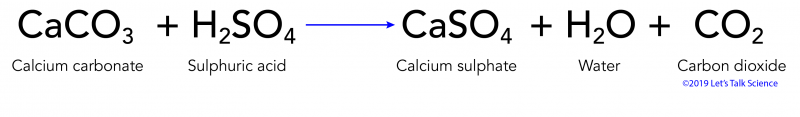
The carbonate content is the primary controlling factor on the pH of the shale-acid system over time. Incompatible with acids alum ammonium salts fluorine magnesium. CaCO 3 H 2 SO 4 CaSO 4 H 2 OCO 2.
Calcium carbonate CaCO3 is an insoluble salt which means it dissolves only slightly in water. Well HCl is an acid and it reacts with the metal carbonate Na2CO3 to give CO2 carbon dioxide H2O and NaCl sodium chloride. On reacting with dilute acids calcium carbonate gives carbon dioxide.
The more Carbonate content in the shales the higher pH buffering capacity. The pH of the solution will be monitored as the HCl is added with a pH probe attached to a CBL. Reacts with acids and acidic salts to generate.
When calcium carbonate precipitate exist in water that solution become weak basic due to presence of carbonate ion. In this reaction sodium carbonate reacts with excess hydrochloric acid to form sodium chloride water and carbon dioxide. The flask contains 20 g of calcium carbonate chips and the simulation is paused when it is loaded up.
CO 2 H 2 O H 2 CO 3 The predominant species are simply loosely hydrated CO 2 molecules. The acidity pKa value of sodium carbonate is 1033. Calcium carbonate occurs naturally as chalk limestone and marble.
In shales with abundant of carbonates the released Fe 2 is quickly oxidized to Fe 3 and remains in proximity to the point source which is mainly pyrite. Add water to the shoulder of the bottle transfer from another bottle Shake for another minute and label Part 1B Weigh 03500 g to 04500 g of Sodium Carbonate into 4 separate 250 mL Erlenmeyer flasks Use wax paper to. Generally acids react with metal carbonates to give.
Acid carbonate carbon dioxide salt water When carbon dioxide reacts bubbles fizz and lime water will turn cloudy. As an antacid calcium carbonate is indicated for heartburn caused by gastroesophageal reflux disease GERD NSAID upper gastrointestinal mucosal damage duodenal and gastric ulcers biliary reflux stress gastritis exocrine pancreatic insufficiency bile acid-mediated diarrhea Non-ulcer dyspepsia and urinary alkalization. What happens when sodium carbonate reacts with hydrochloric acid.
These are evidence of carbon dioxide reacting in a solution. It is used to increase the volume in baking. Acid carbonate salt carbon dioxide water.
Carbonate that required 3901 mL of the titrant HCl to reach the second equivalence point. In order to find the compound is an acid or a base one has to consider the ions that are dissolved in water and their ability to accept or donate a hydrogen ion. Serena last name deleted for privacy by Editor - - Auckland New Zealand.
When aqueous HCl is added carbonate is converted to carbon dioxide and alkalinity of the solution decreases. CaCO 3 2HC l CaCl 2 H 2 O CO 2. It is a conjugate base of a hydrogencarbonate.
Sulfuric acid reacts with sodium hydrogen carbonate to form sodium sulfate carbon dioxide and a water molecule. Carbonic acid and carbonate salts. Carbonate is a carbon oxoanion.
The photo shows the. Sodium Carbonate and Hydrochloric Acid Reaction Na.

Carbonic Acid Formation In The Atmosphere Processing Of Mineral Dust Download Scientific Diagram
Carboxylic Acids Easy Exam Revision Notes For Gsce Chemistry

Chemistry Metal Carbonate And Hygrodencarbonates Acids Bases And Salts Part 2 English Youtube

How Acids React With Metal Carbonates Cbse Class 10 Chemistry Notes Youtube
Double Replacement Acid Carbonate Reactions Write Dissociation Equations
Name The Gas Evolved When A Metal Carbonate Or Metal Hydrogen Carbonate Reacts With Acids Explain The Chemical Reaction From Science Acids Bases And Salts Class 10 Haryana Board English Medium
Metal Carbonates And Acids Activity
What Is Acid Rain Let S Talk Science

Question 24 Mcqs From Ncert Exemplar

Acid Metal Carbonate Acids Bases Alkalis Chemistry Fuseschool Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube

Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube

Posting Komentar untuk " "