Carbonate And Bicarbonate Titration
This experiment is carried out to determine the percentage of calcium carbonate CaCO3 in the toothpaste provided with the experimental technique known as back titration. The sodium bicarbonate was titrated by incrementally adding 1 mL of sulfuric acid to 100 mL of the sodium bicarbonate in a 150 mL beaker while being stirred on a stir plate with a stir bar.

During The Titration Of Sodium Carbonate With H Cl The Dissolved Carbonate Ion Will Exist In Three Different Forms Co 3 2 H Co 3 1 And H 2 Co 3 During Which Part Of The Titration Initial
This method quantifies bicarbonate HCO 3- and carbonate CO 3 2- levels in a soil water extract such as from saturated paste extractQuantitation is by titration with 0025 N H 2 SO 4The measurement should be made immediately due to the potential of the extract being super- saturated.

Carbonate and bicarbonate titration. Titration of Sodium Carbonate With Sodium Bicarbonate After Second End Point for Methyl Orange. Lakes in watersheds that have carbonate minerals have high alkalinity and are not susceptible to acidification. Calcium Carbonate Back Titration.
For this reason a SIA titration system has been developed for the determination of total alkalinity by titration with hydrochloric acid containing methyl orange as indicator end point of pH 45 and for the determination of alkalinity due to carbonate ions by titration with hydrochloric acid with phenolphthalein as indicator end point of pH 83. Summary of Contract Required Detection Limits Holding Times and Preservation for Alkalinity Analytical Parameter Contract Required Detection Limit CRDL Technical and Contract Holding Times Preservation Low-Level Alkalinity. For Bicarbonate add few drops of mixed.
In this experiment a solution of Na2CO3 will be titrated with a solution of HCl. The double value of titrant is the epm of carbonate if sample is taken 20 ml. Titration to the bromocresol green end-point ensures that all of the carbonate and bicarbonate have been converted to H 2CO 3 and then to H.
By titrating a sodium bicarbonate standard the concentration of the sulfuric acid titrant was determined. The shape of the pH titration curve will be observed and the Kb values for the base will be determined. Multiply the double of titrant volume by 30 you will get ppm of carbonate.
1 To prepare a standard 01 M HCl solution 2 To determine the amount of bicarbonate and the original amount of the carbonate in the unknown --- mixture. CARBONATE BICARBONATE and TOTAL ALKALINITY Standard Methods 2320 Titration Method Table 1. The average errors of the bicarbonate concentrations in each series of titration are shown in Table 1.
D Bicarbonate and Hydroxide alkalinities cannot be present together e All hydroxide alkalinity is neutralized by pH 100 f all Carbonates are converted to bicarbonates by pH 83 Group Result of Hydroxide Carbonate Bicarbonate Titration Alkalinity Alkalinity Alkalinity A P 0 0 0 T initial pH 83 B P 05T 0 2P 0 C P T T 0 0. A known mass of toothpaste is neutralised with a known concentration. Calcium carbonate in water with a fixed partial pressure of carbon dioxide.
53332 Add 20ml of 150gL solution of potassium carbonate and heat it to boiling on burner. Volume_hcl Volume Of Sodium Carbonate2Volume Of Sodium Bicarbonate Go. Volume_hcl Volume Of Sodium Hydroxide Volume Of Sodium Carbonate2 Go.
The first equivalence point corresponds to the added potassium carbonate while the second one corresponds to the sum of potassium. For all of the samples titrations also determined the bi carbonate concentration which is theoretically equal to zero in VFA standard solutions. HC03- H H2CO3 C032- 2H H2CO3 A separate aliquot of unknown is treated with excess standard NaOH to convert HCO3- to CO32-.
For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system. The titration curves for potassium bicarbonate alone clearly exhibit only one EP for potassium bicarbonate while the titration curves for the solution withpotassium bicarbonate and potassium carbonate have two EPs. The carbonate and carbonic acid equivalence points may be determined either by titration using indicators or by pH titration.
Titration of Sodium Hydroxide and Sodium Carbonate Phenolphthalein. CO 3 2 HCO 3 3H 2H 2O 2CO 2. 2 mgL 20 mgL.
First total alkalinity moles of bicarbonate moles of carbonate is measured by titrating the mixture with standard HCI to a methyl orange end point. Ba2 CO32- BaCO3 s The. 53331 Take a test tube and add 20ml of solution S.
Lab report 2 determination of carbonate and bicarbonate in a mixture by acid-base titration objective. Carbonate minerals such as limestone and dolomite weather rapidly to produce bicarbonate ions. K CaCO Ca CO o CaCO 3 2 - 3 2 3 19.
220 Bicarbonate And Carbonate In Saturated Paste Extract Saturated Paste Extract. The pH of the titration mixture was monitored. Titration of Sodium Carbonate with Hydrochloric Acid.
53334 No ppt is formed. Another titration using phenolphthalein as indicator will give the concentration of carbonate. HCO 3 CO 3 Summary.
53333 Observe the changes. The total amount of carbonate and bicarbonate in the mixture is determined by titration against standard hydrochloric acid using methyl orange as indicator. HC03- OH- CO32- H2O Then all the carbonate is precipitated with BaCl2.
Determination of sodium carbonate and sodium bicarbonate in mixed base by acid-base titration combined with inductively coupled plasma atomic emission spectrometry. The pH of the solution will be monitored as the HCl is added with a pH probe attached to a CBL. The analytical reaction that will happen during your titration is 2 H CO 3 2-H 2CO 3 H 2O CO 2 g Cl-and Na are spectators Note that each mole of carbonate requires two moles of acid for complete titration.
A back titration is also known as indirect titration. The first end point determined in the pH range 83-10 represents the completionequivalence point or stoichiometric end point of the following reaction. 53335 Add 40ml of potassium pyroantimonate solution again heat it.
This is the solubility product of calcium carbonate. CaMgCO32 2H U Ca2 Mg2 2HCO 3 1.

Titration Curve For A Mixture Of Hydroxide Silicate And Carbonate The Download Scientific Diagram

Titration Of A Mixture Of Carbonate And Bicarbonate

Titration Stoichiometry Openstax Cnx
What Is The Reason Why Phenolphthalein Cannot Show The Endpoint For The Complete Titration Of Na2co3 With Only Phenolphthalein Quora
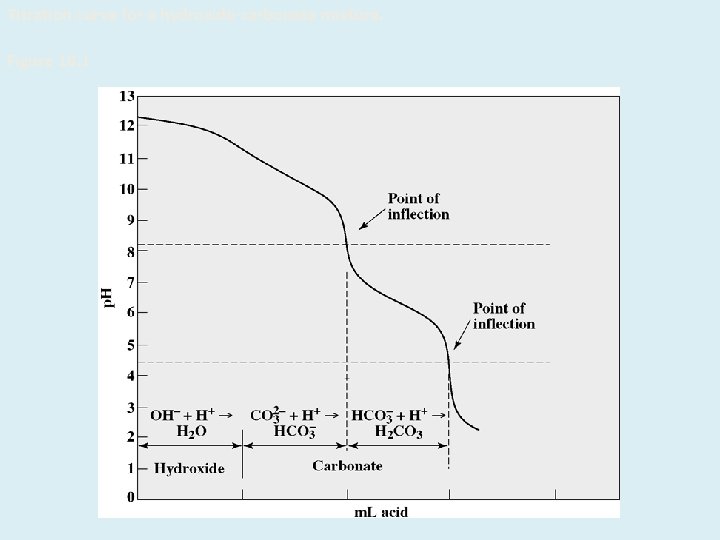
Enve 201 Environmental Engineering Chemistry 1 Alkalinity Dr

Carbonate Bicarbonate Buffer Ph 9 2 To 10 6 Preparation Guide And Recipe Recipe Can Be Automatically Scaled By Entering D Preparation Recipes Chemistry Labs

The Theoretical Titration Curves And Gran Functions For Titration Of Download Scientific Diagram

The Titration Curve And Gran Functions For Titration Of The A Download Scientific Diagram

The Titration Curve And Gran Functions For Titration Of The A Download Scientific Diagram
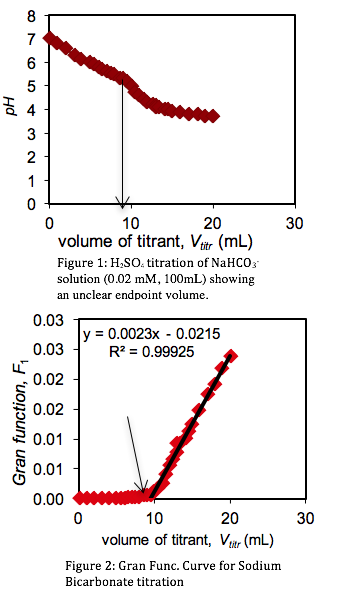
Carbonate Alkalinity Determination Odinity

Conductivity Titration Of 49 75 Mm Naoh With Co 2 Delivered Through A Download Scientific Diagram


Posting Komentar untuk "Carbonate And Bicarbonate Titration"