Copper 1 Carbonate Formula
Silicon water silicon dioxide hydrogen. It is a non-flammable compound.

1 St E Transferred 2 Nd Ions Form 3 Rd Opposite Ions Attract Bond Is Formed By Emf Compound Is Made Ionic Compounds Ppt Download
This reaction can also be viewed as a double displacement reaction too.

Copper 1 carbonate formula. To convert from copper carbonate to copper oxide. Find Sigma-Aldrich-207896 MSDS related peer-reviewed papers technical documents similar products more at Sigma-Aldrich. This was a precipitation reaction because two solutions Cu NO32 and Na2CO3 were mixed together and a insoluble solid CuCO3 was produced.
The addition of heat energy causes chemical bonds to break and the reactant decomposes into two products. Its color can be different shades of blue or green depending on the purity and the presence of other basic copper carbonates which are usually present in. Lets understand the nature of this reaction and what will be the final product of this reaction.
Cu2CO3 Cu2O CO2. CopperII carbonate heat rarr copperII oxide carbon dioxide. Iron oxygen iron III oxide 4Fe 3O 2 2Fe 2O 3 64.
The Cu NO32 solution was pale blue while the Na2CO3 solution was clearcolourless. CoPPErI CaRbONaTe molecular weight. CuCO_3s heat rarr CuOs CO_2g.
The Copper II Carbonate Formula is CuCO 3Further it is an ionic solid compound which consists of copper II cations Cu 2 and carbonate anions CO 2 3It is not that easy to be found because it is quite difficult to prepare. Carbonic acid dicopper I SCHEMBL221807. Copper I oxide hydrochloric acid copper I chloride water Cu 2O 2HCl 2CuCl H 2O 62.
It is insoluable in water. The precipitate formed is copper I carbonate. Lets us learn the chemical formula and other details about Copper II Carbonate.
Basic copper carbonate is a chemical compound more properly called copperII carbonate hydroxide. CopperII carbonate or cupric carbonate is a chemical compound with formula CuCO 3At ambient temperatures it is an ionic solid a salt consisting of copperII cations Cu 2 and carbonate anions CO 2 3. To convert from copper oxide black to copper carbonate.
CopperII carbonate CAS Number. Monoisotopic mass 121942902 Da. The balanced chemical equation follows.
644 100 0644 amount of copper carbonate amount of copper oxide. 6 moles CoPPErI CaRbONaTe to grams 424307497 grams 7 moles CoPPErI CaRbONaTe to grams 495025413 grams 8 moles CoPPErI CaRbONaTe to grams 56574333 grams. Average mass 122590 Da.
Copper II Carbonate is also referred to as Cupric Carbonate. Molecular Formula CHCuO 3. Average mass 124563 Da.
As shown in the equation above. The name most commonly refers to the compound with formula Cu 2 CO 3 OH 2. Magnesium bicarbonate hydrochloric acid magnesium chloride water carbon dioxide MgHCO 3 2 2HCl MgCl 2 2H 2O 2CO 2 63.
This compound is rarely encountered because it is difficult to prepare and readily reacts with water moisture from the air. Copper II Carbonate is a grey colour ionic solid compound that contains copper II cations Cu2 and carbonate CO 32- anions. When potassium carbonate reacts with copper I fluoride it leads to the formation of solid copper I carbonate and potassium fluoride solution.
The new substance formed was CUCO3 an insoluble precipitate. Further it is essentially a chemical compound. The heating of solid copperII carbonate is a thermal decomposition reaction.
Molar mass of CoPPErI CaRbONaTe 707179162 gmol Convert grams CoPPErI CaRbONaTe to moles or moles CoPPErI CaRbONaTe to grams. It is an ionic compound a salt consisting of the ions copperII Cu 2 carbonate CO 2 3 and hydroxide OH. Copper carbonate CuCO3 or CCuO3 CID 14452 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
CopperII carbonate basic reagent grade. 36 Votes Copper II carbonate is a powdery blue-green compound that is insoluble in water. 415 57 Views.
Cu2CO3 is a light green powder at room temperature. The terms copper carbonate copperII carbonate and. Molecular Formula C 2 H 3 CuO 2.
Monoisotopic mass 123922173 Da. 10000 644 155 amount of copper oxide amount of copper carbonate. Its melting point is 200 C 392 F boiling point 290 C 554 F density 39 gcm3.
The reaction between copper carbonate and sulphuric acid will be a neutralization reactionCopper carbonate will acts as a base however sulphuric acid is an acid.

How To Write The Formula For Copper I Carbonate Youtube
Copper Ii Carbonate Ccuo3 Chemspider
Solved D Copper 1 Carbonate For Each Of The Following Chegg Com
Copper Carbonate Cuco3 Pubchem

How To Balance Cuco3 Cuo Co2 Decomposition Of Copper Ii Carbonate Youtube

1 Chemical Bonding Process Of Joining Atoms Together Chemical Bond Force Holding Atoms Together Within A Substance Why Bonding Creates A New Arrangement Ppt Download

How To Write The Formula For Copper I Carbonate Youtube

Solved 1 76 G Of Copper Ii Carbonate Was Heated In A Chegg Com
Copper 1 Hydrogen Carbonate Chcuo3 Chemspider

How To Write The Formula For Copper Ii Carbonate Youtube

Copper Carbonate Formula Copper I Carbonate Formula Copper Ii Carbonate Formula Youtube

Naming Balancing Drills 1 Name The Compound 1 Mg No 3 2 2 Ca 3 N 2 3 Mnc 2 O Ppt Download

Type Of Reaction For Cuco3 Cuo Co2 Youtube

Negative Ion Formula In Compoundcharge Oxide Hydroxide Nitrate No 3 1 Sulphate Carbonate Ppt Download
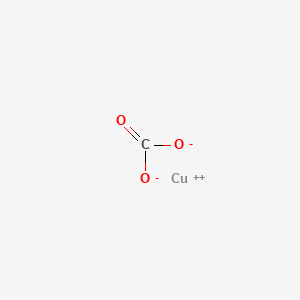
Posting Komentar untuk "Copper 1 Carbonate Formula"