Sodium Carbonate X
Thus 02 N Na2CO3 022 M Na2CO3 01. Sodium Carbonate is a sodium salt of carbonic acid soluble in water.
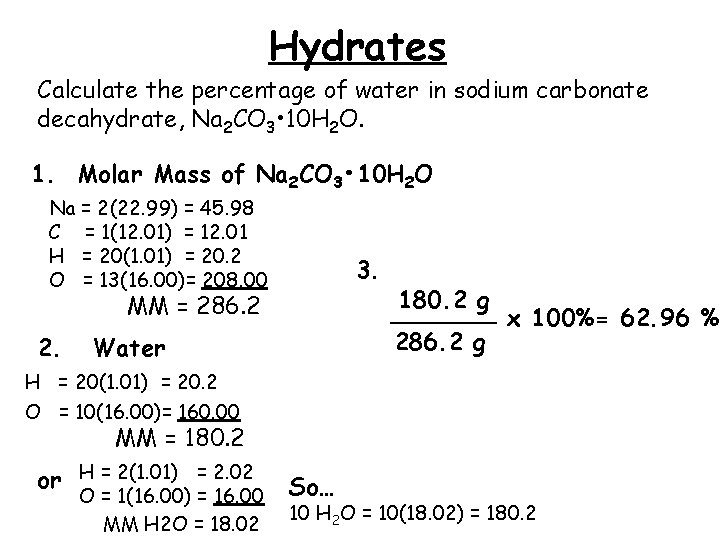
Percent Composition Empirical Formulas Molecular Formulas Percent Composition
The first method I will use to determine x will involve heating the crystals.
Sodium carbonate x. Crystallisation in hydrated sodium carbonate Theory 2 Hydrated sodium carbonate has the formula Na 2CO 3xH 2O where x is the number of molecules of water of crystallisation present. Its water solubility is 215 gl at 20C. The trend pattern remained consistent with.
3 This is the part Im unsure about. Powders with a lower pH around 10 will have more STPP as. When treated with acid it is converted into sodium bicarbonate.
In general imports posted a notable increase. Calcined soda-13 C Carbonic acid disodium salt-13 C 13 C Labeled sodium carbonate Soda ash-13 C. In this experiment x is determined by titration of a solution made using hydrated sodium carbonate with a standard solution of hydrochloric acid.
497-19-8 Anhydrous 5968-11-6 Monohydrate 6132-02-1 Decahydrate. When dissolved in water sodium carbonate forms carbonic acid and sodium hydroxide. Where to buy Sodium Carbonate in the USA.
Washing soda soda ash and soda crystals. In value terms sodium carbonate imports contracted notably to X in 2020. The total output value increased at an average annual rate of X over the period from 2007 to 2020.
Na 2 CO 3. 2 Since the molar concentrations of the dissociation products HCO3- and OH- are the same we can simplify Eqn. Stripping CO 2 gas from the withdrawn aqueous mining solution to convert sodium bicarbonate dissolved therein to sodium carbonate.
2 I calculated the molar mass of sodium carbonate and sodium bicarbonate. Sodium hydrogen carbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO3. Pure sodium carbonate is a white odorless powder that is hygroscopic absorbs moisture from the air and that forms a moderately basic solution in water.
The most prominent rate of growth was recorded in 2011 with an increase of X against the previous year. Sodium carbonate has a melting point of 851C it decomposes when heated and therefore a boiling point can not be determined. Higher cost products will use STPP as primary ingredient.
Normality N n factor X Molarity M n factor for salt Na2CO3 is the number of moles of electron involved by 1 mole of the salt. When HCl solution is added slowly to the Na 2 CO 3 solution reaction happens in two stages. It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3.
It is also known as Soda crystals soda ash washing soda. Sodium carbonate comes with the chemical formula Na_2Co_3. Aqueous sodium carbonate titration with HCl solution.
Sodium bicarbonate comes with sodium acid and hydrogen. A process for the production of soda ash by withdrawing an aqueous mining solution containing dissolved sodium carbonate and at least about 1 wt sodium bicarbonate from an underground alkali source. Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property.
Sodium carbonate is an inorganic salt and therefore the vapour pressure can be considered negligible. Here there are 2 electron involved so the n factor for Na2CO3 is 2. Sodium carbonate is made up of sodium and acid.
Sodium Carbonate also known as. Sodium bicarbonate IUPAC name. Sodium carbonate is a basic salt which is made of a strong baseNaOH and weak acid H2CO3.
It ionizes into 2Na and CO32- which in water then becomes CO2 and H2O. When dilute HCl is slowly added first sodium bicarbonate and sodium chloride are formed. MM of sodium carbonate 10599 g m o l 10599 g m o l.
Higher pH products have sodium metasilicate as well. The base dissociation constant Kb of sodium carbonate is 0000212766 so we can write this equation- HCO3-OH- CO3-2 0000212766 Eqn. Sodium carbonate commonly occurs as a crystalline decahydrate Na 2 CO 3 10H 2 O which readily effloresces to form a white powder the monohydrate Na 2 CO 3 H 2 O.
Sodium carbonate is a diazonium salt of carbonic acid with chemical formula Na2CO3. MM of sodium bicarbonate 8401 g m o l 8401 g m o l. In value terms sodium carbonate production dropped slightly to X in 2020 estimated in export prices.
The percentages in a formula are usually determined by cost with sodium carbonate X costlb sodium metasilicate 15 X and STPP 4X. How to calculate the ratio of sodium carbonate and sodium bicarbonate in x Molar sodium carbonate-sodium biocarbonate buffer. Aqueous sodium carbonate is a weak basic solution and HCl is a strong acid.
Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. Low cost powdered products will use sodium carbonate as primary ingredient. Sodium bicarbonate formula is given as NaHCo_3.
Na2CO3x H2O is the formula for hydrated Sodium Carbonate where x represents the number of water molecules present in the crystals. The only thing you still have to calculate is the molar mass of the initial compound. In this video we will describe the equation Na2CO3 H2O and write what happens when Na2CO3 is dissolved in waterWhen Na2CO3 is dissolved in H2O water it.
As a strong base sodium hydroxide neutralizes gastric acid thereby acting as an antacid. For instance I wish to. Imports peaked at X in 2019 and then contracted notably in the following year.
The average particle size diameter d 50 of light sodium carbonate is in the. M m n 35 M m n 35 g 001225 001225. Na2CO3 sodium carbonate is a polar compound.
This inorganic compound is water-soluble and when dissolved in water it forms carbonic acid and sodium hydroxide. You have found that the amount of N a X 2 C O X 3 N a X 2 C O X 3 is n 001225 n 001225 mol. Na 2 CO 3 HCl NaCl NaHCO 3.
Co-crystallizing sodium carbonate monohydrate and sodium.
Sodium Carbonate 99 Hplc Selleck Others Chemical

Determine The Formula Of Hydrated Sodium Carbonate Gcse Science Marked By Teachers Com

Sodium Carbonate Decahydrate Cas 6132 02 1 Scbt Santa Cruz Biotechnology
Sodium Carbonate Reacts With Dil H2so4 To Give The Respective Salt Water And Carbon Dioxide Sarthaks Econnect Largest Online Education Community

Molarity And Stoichiometry 1 Calculate The Number Of Grams Of Sodium Carbonate That Are Required To React Fully With Ml Of M Hcl Na 2 Co Ppt Download

Sodium Carbonate An Overview Sciencedirect Topics

Moles And Solutions Specifications Moles And Solutions Calculate The Amount Of Substance In Moles Using Solution Volume And Concentration Ppt Download

Percent Composition Empirical Formulas Molecular Formulas Percent Composition

Determine The Formula Of Hydrated Sodium Carbonate Gcse Science Marked By Teachers Com
Sodium Carbonate Na2co3 Pubchem
Washing Soda Has The Formula Na2co3 10h2o What Mass Of Anhydrous Sodium Carbonate Is Left When All The Water Of Crystallization Is Expelled By Heating 57 2 G Of Washing Soda

In An Experiment To Determine The Percentage Purity Of The Sample Of Sodium Carbonate Produced In The Solvay Process 2 15 G Of The Sample Reacted Completely With

57 2g Of Hydrated Sodium Carbonate Na Sub 2 Sub Co Sub 3 Sub Xh Sub 2 Sub O Were Dissolved In Water And The Solution Made To One Litre 20cm Sub 3 Sub Of 0 5m Hcl Recated With 25cm Sub 3 Sub Of
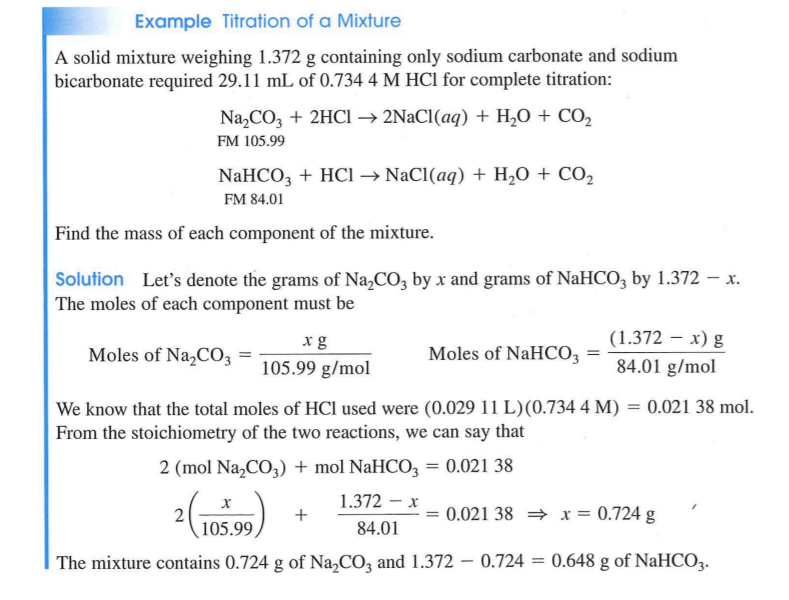
Solved Example Titration Of A Mixture A Solid Mixture Chegg Com

Posting Komentar untuk "Sodium Carbonate X"