Carbonate Base Formula
Iron III carbonate also known as ferric carbonate is an inorganic salt that is found in some minerals. For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system.
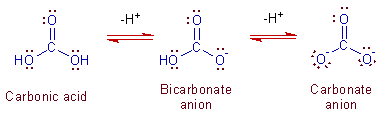
Carbonates Bicarbonates Formula Examples Structure Reactions Preparation
The only place CO3 can get hydrogen ions from is water.
Carbonate base formula. HBr aq KOH aq H 2 O l KBr aq Hydrochloric acid reacts with ammonia to form ammonium chloride a salt. Apart from Li2CO3 alkaline metal carbonates are stable. A carbonate is a salt of carbonic acid H.
The molar mass is 29172 g mol-1. CO 3 2-s 2H aq CO 2 g H 2 Ol This time the spectator ions you are left with are copperII ions and sulfate ions in solution - blue copperII sulfate. The name of the most common form of the acid consists of the nonmetal root name with the -ic ending.
It is itself a fairly strong base and has no hydrogens to contribute to another base nor does the carbon of the carbonyl group Ie at the CO have the ab. How many atoms are in aluminum carbonate. Metal carbonates from group 1 on the Periodic Table will have the formula M 2 CO 3.
That is distinguished by the presence of the carbonate ion a polyatomic ion. In carbonate ionthe oxidation number of carbon is 4 and the oxidation number of O is -2The net charge of carbonate is -2. Iron III carbonate chemical formula is Fe2CO33.
Carbonates are made from reaction between carbonic acid aqueous carbon dioxide and a base or alkali. To know whether the MCO 3 or M 2 CO 3 will be the correct formula there are two simple rules to remember. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-.
This inorganic compound is water-soluble and when dissolved in water it forms carbonic acid and sodium hydroxide. It may be a moderately strong base. Carbonate salts are generally considered weak bases and they turn litmus paper blue.
Carbonate rock weathering of is major global factor in natural water acid-base chemistry. Potassium bromide is made up of K cations from the base KOH and Br anions from the acid HBr. CaCO 3-CO 2-H 2 O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide.
Alkali metals are often mined within the sort of Na2CO3 washing soda. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms where the use of chalk a form of CaCO 3 is found. It mostly used as a cleansing agent for domestic purposes.
Ternary acids commonly contain hydrogen a nonmetal and oxygen. CaCO 3 sthe principle mineral in limestonecan be reformulated as a sum of oxides. A carbonate ester an organic molecule bearing the carbonate group C O O.
Soda ash also known as sodium carbonate Na2CO3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate-bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash. When a carbonate reacts with an acid carbon dioxide is produced the gas that turns limewater milky and water which boils at about 100C is also produced. Formula for Carbonate.
De-code the word equation you wrote to try to re-create Chriss experiment. What is the chemical name for Al2 CO3 3. CaO CO 2 s.
In chemistry a carbonate is an ion consisting of one carbon and three oxygen atoms or a compound that contains this species as its anion. I n carbonateone oxygen is bonded to carbon with double bond and two other oxygens are bonded to carbon with single bondSo Structural formula for carbonate is given below. In the formula M represents a metal atom C represents a carbon atom and O represents an oxygen atom.
-water system unless other sources of base are added. Carbonate is a moderately strong base. Alkali metals can be mined in the form.
The reactants are potassium carbonate and sulfuric acid so Chris added sulfuric acid to potassium carbonate. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
Carbonates are readily decomposed by acids. It is a unstable compound. The chemical formula for calcite.
Ammonium chloride is made up of NH 4 cations from the base NH 3 and Cl anions from the acid HCl. The molecular formula for the carbonate ion is CO 3 2-. Heres why carbonate is a salt with a minus two 2 charge carrying the formula CO32-.
Except for Li2CO3 alkali metal carbonates are thermally stable. Alternatively the term may be used as a. It is commonly known as washing soda or soda ash.
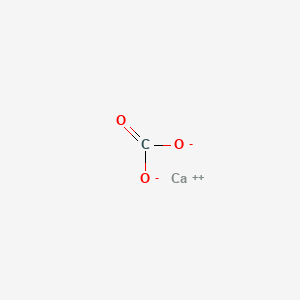
The carbonate formula is CO. Sodium carbonate is a diazonium salt of carbonic acid with chemical formula Na2CO3. Calcium carbonate is a chemical compound with the formula Ca CO 3.
A metal carbonate has the general formula MCO 3 or M 2 CO 3. It is also known as Soda crystals soda ash washing soda. Formulas of Ternary Acids.
Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. When you put sodium carbonate Na2CO3 in water it produces 2 sodium ions and one carbonate ion CO3 with a charge of 2-. It appears as a white solid and soluble in water.
The acid containing one less oxygen atom than the most common form is designated by the -ous ending. Sodium carbonate is an inorganic compound composed of sodium salt and a carbonate salt having the chemical formula Na 2 CO 3. It is a conjugate base of a hydrogencarbonate.
In its pure form it is white powder and odourless. Carbonate group C O O 2. It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells snail shells seashells and pearls.
The ionic equation showing the reaction between the carbonate and hydrogen ions is exactly the same as before - except of course that we know copperII carbonate is a solid. They have the formula MxCO3y eg. And if it takes a hydrogen from water H2O there will be.

Identify The Acid And Base Which Form Sodium Hydrogen Carbonate Write Chemical Youtube
Nickel Ii Carbonate Cnio3 Chemspider

Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube

11 7 Reactions Of Acids And Bases Ppt Download
Copper Ii Carbonate Hydroxide Ch2cu2o5 Chemspider

Potassium Carbonate Definition Uses Formula Physical Properties
Calcium Carbonate Caco3 Pubchem

Equation For Sodium Carbonate Dissolving In Water Na2co3 H2o Youtube

Chemistry Metal Carbonate And Hygrodencarbonates Acids Bases And Salts Part 2 English Youtube

Names Chemical Formulas And K Values For Major Carbonate Minerals Download Table

Which Formula Can Be Used To Calculate The Exact Hydronium Concentration Present In Sodium Hydrogen Carbonate Solution Chemistry Stack Exchange

Chemical Formula Of Various Magnesium Calcium Carbonates The Numbers Download Table

Chemical Formula Of Various Magnesium Calcium Carbonates The Numbers Download Table
Posting Komentar untuk "Carbonate Base Formula"