Carbonate Buffer Ph Range
The other 1 is dissolved Carbon Dioxide. The normal present day pH of seawater is more on the basic side between 79 90.

How Do I Prepare Carbonate Buffer
As you probably noticed the chemical reaction has multiple steps and can go in both directions.

Carbonate buffer ph range. The pH of seawater is dependent on which of these species is the most predominant. 3 Adjust to 1000 ml with distilled water. Has three pKa values that give it three buffering ranges.
As calculated by the HendersonHasselbalch equation in order to maintain a normal pH of 74 in the blood whereby the pKa of carbonic acid is 61 at physiological temperature a 201 bicarbonate to carbonic acid must constantly be maintained. Recipe can be automatically scaled by entering desired final volume. For reaching 85 you will need a large amount of bicarbonate and very small amount of carbonate.
In fact the pH range of effectiveness is probably 51 71 for the bicarbonate buffer system. The bicarbonate acts like a buffer in the ocean. Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings.
Note that the bicarbonate-carbonate buffer works best in the pH range 92-108. Chloride Buffer pH 20. A 1001 ratio so most buffer systems will work over a total range of about 4 pH units.
Carbonate-Bicarbonate Buffer pH 92 to 106 Preparation. 32 rows Citric Acid Sodium Citrate Buffer Preparation pH 30-62. Oxalic Acid C.
Carbonate Buffer pH 97. 01M Sodium Bicarbonate-Sodium Carbonate Buffer pH 90 UPR16490 250 ml - Applications. Coating protein oon microplates Citrate all Tech Sheet pKa1313pH range22-65 pKa2476pH range30-62 pKa3640pH range55-72 working global pH range 3-62.
PH range 57 to 72 Dissolve 192g of MES free acid MW 1952 in 900mL of pure water. Bicarbonate is better described as a CO2 transport mechanism and not as a buffer protons combine with hydrogen ions which are at equilibrium with carbonic acid H 2 CO3 water and CO 2. There are other uses but thats general knowledge of chemical solutions not a physiological phenomenon or system.
2 Titrate to pH 69 with 3 M HCl. Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings. Carbonate ion concentrations increase with increasing pH and when more CO2 dissolves in seawater.
Sodium Acetate Acetic Acid. 67 rows effective pH range. Buffers in the pH.
Major buffers that exist in the rumen Counotte et al 1979 include HCO3pKa 380 carbonate pKa 1025 phosphate pKa 212 721 and 1232 acetate pKa 476 propionate pKa 487 butyrate pKa 482 andlactate pKa 386. What this means is that this system is very resistant to changes in pH. Dissolve 657 g of potassium chloride in water add 1190 ml of 01 M hydrochloric acid and dilute with water to 1000 ml.
Carbonate buffers are a combination of a weak acid and its conjugate salt relying upon series of reverse reactions to buffer changes in pH. Buffers pKa range. Bicarbonate HCO3 and carbonate CO3 ions.
Nitric Acid - HNO. Notice that there is a gap in buffering between pH 31 and pH 62 for phosphate. Carbonate-Bicarbonate Buffer pH 92 to 106 preparation guide and recipe.
Perchloric Acid HClO. Titrate to desired pH with 1 N HCl or 1 N NaOH and adjust final volume to 1000mL. The balanced equation is below.
15 rows Buffer Preparation Calculator University of Liverpool. 840 Dissolve x g of Na2CO310H2O and y g of NaHCO3 in distilled water according to the pH required and bring the total volume to 100 ml. 02 M solution of anhydrous sodium carbonate 212 g in 1000 ml.
Thus for the carbonate system were worried about here if. Most of these weak acids and bases have pKa that are outside the normal pH range of the rumen. The carbonate and phosphate buffer systemsthese are not in the pH range necessary to buffer blood.
A mixture of sodium bicarbonate and sodium carbonate is a buffer and can be used between pH of about 88 to 106 at 37 o C or about 92 to 108 at 20 o C. They work best of course near the middle of their range. Potassium Chloride KCl 11-18.
To make 1 liter of carbonate buffer add the following. 2862 NaHCO3 MW. 028 M cacodylate buffer pH 69 MATERIALS Sodium cacodylate 3 M hydrochloric acid METHOD 1 Dissolve sodium cacodylate 60 gl in distilled water.
Buffer pKa and pH Range Values For preparation of. Practical limits of column stability require that we truncate the lower range to 20. Carbonatebicarbonate buffer Stock solution A.
Dissolve 84 g of sodium bicarbonate and 106 g of sodium carbonate in sufficient water to produce 500 ml. Hydrochloric Acid - HCl 0-2. 16 g Na2CO3 15 mM final 29 g NaHCO3 35 mM final 02 g NaN3 31 mM final.
At this pH the HCO3 ions predominate. This question now b. 11.
This homeostasis is mainly mediated by pH sensors in the medulla oblongata of the brain and probably in the kidneys linked via negative feedback loops to. 01 M Sodium carbonate-sodium bicarbonate buffer solutions pH 92-105 at 20C Na2CO310H2O MW. The bicarbonate buffer system is what the body uses.

How Do I Prepare Carbonate Buffer

Effect Of Temperature On Ph Of Phosphate Buffer Download Scientific Diagram
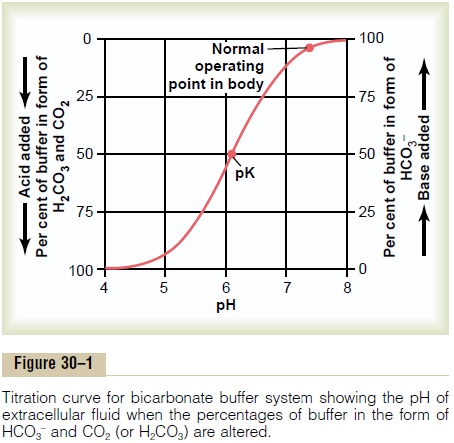
Quantitative Dynamics Of The Bicarbonate Buffer System
The Bicarbonate Buffering System And Titration Curves Practice Khan Academy
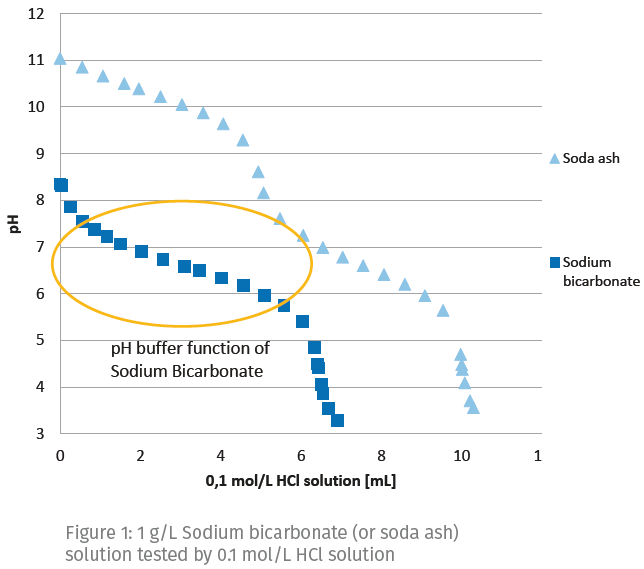
Sodium Bicarbonate A Multipurpose Ingredient For Food Applications Asia Pacific Food Industry

Pdf A Sodium Carbonate Bicarbonate Buffer For Alkaline Phosphatases Semantic Scholar
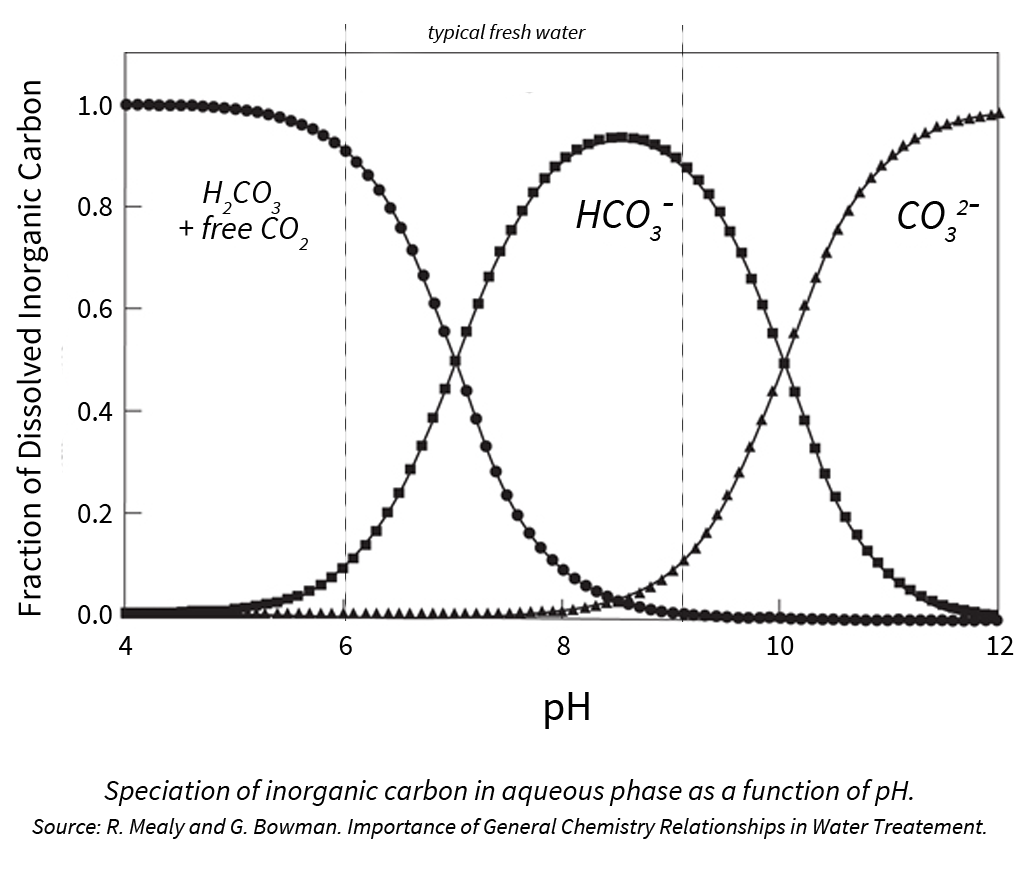
Adjusting Irrigation Water Bicarbonates Vs Ph Master Plant Prod Inc
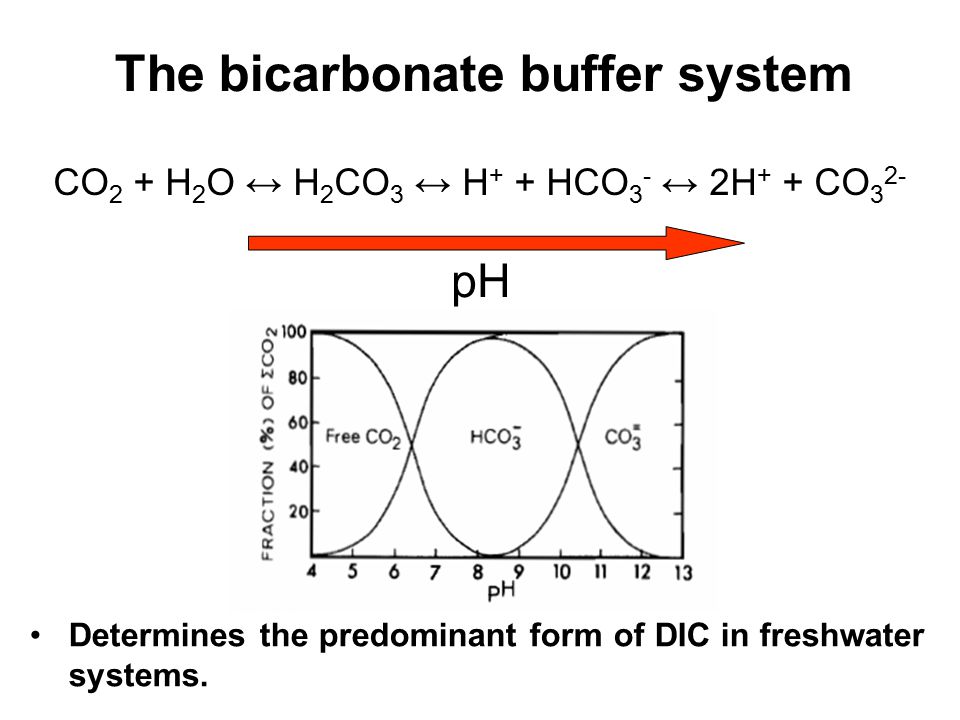
Lecture Goals To Review How Ph And Alkalinity Work Ppt Video Online Download

Acid And Base Balance Department Of Biochemistry 1



Posting Komentar untuk "Carbonate Buffer Ph Range"