Carbonate Oxidation State
For example in case of carbonate ion CO3 2 the oxidation numbers of one carbon atom and 3 oxygen atoms will be equal to -2. Applying this rule oxidation numbers of any element can be calculated in a molecule or an ion.

How To Find The Oxidation Number For Fe In Fe2 Co3 3 Youtube
The oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero.

Carbonate oxidation state. For example in case of carbonate ion CO32 the oxidation numbers of one carbon atom and 3 oxygen atoms will be equal to -2. Were being asked to determine the oxidation state of carbon in CO 3 2 carbonate ion. For an atom in its elemental form Zn Cl 2 C graphite etc OS.
To find the correct oxidation state of C in CaCO3 Calcium carbonate and each element in the compound we use a few rules and some simple mathFirst since. The oxidation state of oxygen is always -2. What is the oxidation state of carbonate.
Give the oxidation number for the specified atom in the following compounds. Applying this rule oxidation numbers of any element can be calculated in a molecule or an ion. This means that the oxidation number of the carbon must be 4.
While fully ionic bonds are not found in nature many bonds exhibit strong ionicity making. What is the oxidation number of carbon in carbonate. Hydrogen has a 1 oxidation state.
So carbon can have a range of oxidation states from minus four to plus four when youre talking about carbon with four bonds. Applying this rule oxidation numbers of. On the basis of XRD and elemental compositions analyses the metallic Ta is firstly oxidized to Ta 2 O 5 by CO 2 released from calcium carbonate or ammonium carbonate decomposition then high-oxidation-state tantalum nitride Ta 3 N 5 can be in situ synthesized under ammonia flow during the heating process.
Determine the oxidation number of calcium in calcium carbonate compound. The oxidation state of carbon in CO is 2. I The oxidation state of carbon is determined to be 4 as it has four valence electrons and can readily lose four electrons to attain the nearest noble gas configuration.
ExplanationHydrogen has a 1 oxidation state. 4The oxidation state of carbon in NaHCO3 is 4. Calcium carbonate oxidation numbers.
These high-valent complexes are intermediates. Oxygen normally has an oxidation state of -2. The oxidation state of carbon in NaHCO3 is 4.
What Is The Oxidation State Of An Individual Carbon Atom In Caco3. What is the oxidation state of CO3. Carbon monoxide CO has to have a total oxidation state of zero since the total charge is zero.
7What is the oxidation state of carbon in aluminum carbonate. For now we know that metallic elements will have positive oxidation state whereas nonmetallic element will have negative. Share Tweet Send Chromium Wikimedia Chromium is an element of the 4ᵗʰ period of the 6ᵗʰ group it is located in the side subgroup.
Compared with the existing synthetic. 38 Lec1 Oxidation States of Organic Molecules. Oxidation states of chromium How many oxidation states does chromium have.
And you can also have in between right its possible of course for carbon to have an oxidation state of plus three I just didnt do an example like that in this video. Carbon atoms can exist in nine different oxidation states running from to. It is a conjugate base of a hydrogencarbonate.
Carbonate is CO32- ion Each oxygen has an oxidation state or oxidation number of -2. See the answer. It is possible for different carbon atoms in the.
Sodium has a 1 oxidation state. See the answer See the answer done loading. Calcium carbonate is a chemical compound with the formula CaCO3.
In electrochemical water oxidation processes that are. Enter your answer using no spaces with the sign after the number. W rite the formula for hydrogen carbonate and determine the oxidation number for carbon.
What is the oxidation number of carbon in Na2CO3 Answer. Oxygen has a -2 oxidation state. The rules for oxidation states are as follows.
So in order for the compound to equal 0 carbon has to have an oxidation state of 2. Sodium has a 1 oxidation state. Formula for the sodium carbonate is Na2CO3It is understood that Na atoms has 1 oxidation number and O atoms has a 2- oxidation number.
As a ligand stabilizes transition metal complexes in uncommon high oxidation states. Actually CaCO3 is an ionic compound made by Ca2 and CO32- ions giving rise to a lattice where they regularly alternate In a CO32- ion. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale.
In nature it is only found in the form of compounds for example chromite or crocoite. Salts or ions of the theoretical carbonic acid containing the radical CO2 3. Carbonate is a carbon oxoanion.
It is a carbonate salt a one- carbon compound an iron molecular entity and a carbonate mineral. For example in case of carbonate ion CO3 2 the oxidation numbers of one carbon atom and 3 oxygen atoms will be equal to -2. It is a common substance found in rocks as the minerals calcite and aragonite and is the main component of eggshells snail shells seashells and pearls.
To find the correct oxidations state of C in CO3 2- the Carbonate ion and each element in the ion we use a few rules and some simple mathFirst since th. For neutral molecules the oxidation number of each carbon atom can usually be found by assigning oxidation numbers of and respectively to bonded hydrogen and oxygen atoms then assigning the carbon values so that the total sums to zero. Carbon has an oxidation state of 4.
Ferrous carbonate is a carbonate salt in which the counter-ion is iron in the 2 oxidation state. We have 2Na atoms 2 and 3O atoms 6-. Oxidation state of caco3.

How To Find The Oxidation Number For C In Co3 2 Carbonate Ion Youtube

What Is The Oxidation State Of The Carbon Clutch Prep

How To Find The Oxidation Numbers For K2co3 Potassium Carbonate Youtube

Oxidation And Reduction Ppt Download

What Is The Oxidation State Of The Carbon Clutch Prep

How To Find The Oxidation Number For C In Caco3 Calcium Carbonate Youtube

How To Find The Oxidation Number For C In Caco3 Calcium Carbonate Youtube
How To Find The Oxidation Number For C In Cuco3 Copper Ii Carbonate Youtube

What Is The Oxidation State Of An Individual Carbon Atom In Caco3 Lisbdnet Com

How To Find The Oxidation Number For C In Mgco3 Magnesium Carbonate Youtube

How To Find The Oxidation Number For C In Pbco3 Lead Ii Carbonate Youtube
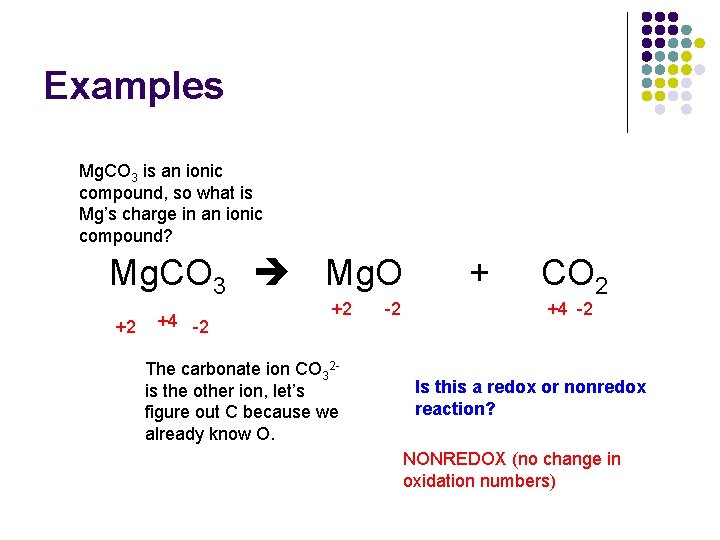
Redox Reactions Chapter 18 O 2 Oxidationreduction Redox

How To Find The Oxidation Number For C In Nahco3 Sodium Hydrogen Carbonate Youtube

What Is A Compound A Substance In Which The Atoms Of Two Or More Different Elements Combine Together Sodium Chloride Nacl Carbon Dioxide Co 2 Calcium Ppt Download
Posting Komentar untuk "Carbonate Oxidation State"