Carbonate Alkalinity Of Water Is Determined By Using Indicators In The Sequence
EXPERIMENT-1 TO DETERMINE THE ALKALINITY IN A SAMPLE OF WATER Alkalinity is a measure of the acid-neutralizing capacity of water and is usually determined by titration against sulfuric acid to the endpoint of the acidbase reaction. Carbonate and carbonic acid equivalence points may be determined either by titration using indicators or by pH titration.

Geological Cross Section Of The Ironstone Bearing Sequence In The Download Scientific Diagram
In these situations water is usually softened by precipitating the CaCO 3 or by using ion exchange softening methods.

Carbonate alkalinity of water is determined by using indicators in the sequence. Can be used to distinguish between three types of alkalinity. Alkalinity is measured by volumetric analysis using a standardized acid titrant. This scale results from the precipitation of calcium carbonate which becomes less water soluble as the tem-perature increases Snoeyink and Jenkins 1980.
Hydroxides carbonates bicarbonates compounds dissolved in water. Alkalinity is usually imparted by the bicarbonate carbonate and hydroxide components of a natural or treated water supply. Here M refers to the pH indicator methyl orange endpoint of about 42 to 45 P alkalinity or Phenolphthalein alkalinity is the measurement of amount carbonate and hydroxide using titrating water sample with acid of a known concentration and using Phenolphthalein indicator.
The carbonate alkalinity and the total alkalinity are useful for the calculations of chemical dosages required in the. Many water samples have little or no phenolphthalein alkalinity and therefore remain colorless after adding this indicator to. The titration is performed volumetrically using phenolphthalein and methyl orange indicators.
Alkalinity is a general water chemistry parameter and can. Stoichiometry of the reaction and number of moles of acid needed to reach the end point the concentration of alkalinity in water is calculated by volumetric methods Alkalinity of water is attributed to the presence of OH- HCO 3. Alkalinity is determined by titration method.
P ½ M. A logarithmic relationship is obtained. The sample is titrated against hydrochloric acid using phenolphthalein and methyl orange as indicators.
The alkalinity of water can be determined by titrating the water s with Standard acid solution HCl. Addition of phenolphthalein gives pink red colour in presence of carbonates and titration with H2So4 converts this carbonates into bicarbonates and decolourise the. Alkalinity determination procedure divided in two parts called P-Alkalinity M-Alkalinity.
In hot water heaters and industrial systems where water is heated. Alkalinity is due to the presence of 6. Alkalinity is present due to HCO3 ion only which can be determined using methyl orange indicator and called methyl orange alkalinity M.
Hydroxide carbonate and bicarbonate alkalinity. P Alkalinity is the alkalinity that is determined by using phenolphthalein indicator. The result can be expressed as mgl CaCO3.
Carbonate alkalinity is determined by titration of the water sample to the phenolphthalein indicator endpoint or approximately a pH of 83. The alkalinity of water is a measure of its capacity to neutralize acids. Titrations can distinguish between three types of alkalinity.
Carbonate alkalinity is determined by titration of the water sample to the phenolphthalein or metacresol purple indicator endpoint approximately pH 83. Total alkalinity is determined by titration of the water. Apparatus used for measuring water alkalinity.
Carbonate bicarbonate and total alkalinity. Total alkalinity is determined by titration of the water sample to the endpoint of the methyl orange. P-alkalinity is measured down to a pH of 83.
Water that has a pH 83 is said to have phenolphthalein alkalinity which is alkalinity due primarily to the presence of carbonate or hydroxide ions. We can quantify the carbonate CO3 and bicarbonate HCO3 concentration and as well as hydro Oxley OH in the water form the analyzed parameter of Alkalinity P P and Alkalinity M M. A carbonate alkalinity is present when the phenolphthalein alkalinity is not zero but less than the total alkalinity b Hydroxide alkalinity is present if the phenolphthalein alkalinity is more than half the total alkalinity c Bicarbonate alkalinity is present if the phenolphthalein alkalinity is less than half the total alkalinity.
The Carbonate and Bicarbonate ions in the sample can be determined by titrating it against standard sulphuric acid using phenolphthalein and methyl orange indicator respectively. The alkalinity of water is determined by titrating the water sample with Standard acid solution HClAlkalinity of water is attributed to the presence ofOHˉCO₃²ˉ and HCO₃ˉions. A fully automated spectrophotometric sequential injection titration system has been developed for the determination of carbonate and hydrogencarbonate in water samples.
Among of them hydroxyl ions carbonates and bi-carbonates are the main factor. It can be determined by using Acid-Base Titration. It is primarily due to salts of weak acids although weak or strong bases may also contribute.
The P alkalinity and M alkalinity of water are determined by how much acid is taken to lower the pH to the specific value. The endpoint pH may be 45 to 51 for total alkalinity and 83 for phenolphthalein alkalinityThe determination of acidity of water can be found by using reagents standard H2SO4 of 002 N phenolphthalein indicator and methyl orange indicator. The endpoint is signalled by a colour change of a pH indicator such as phenolphthalein or methyl orange or by using a pH meter.
It is due to carbonate bicarbonate and hydroxide ions present in water. Alkalinity means acid neutralizing or acid buffering capacity of water. The alkalinity of water can be determined by titrating the water sample with Sulphuric acid of known values of pH volume and concentrations.
Alkalinity has two types -Phenolphthalein alkalinity denoted by P and the Total Alkalinity denoted by T. Using phenolphthalein indicator neutralization reaches upto HCO3 but using methyl orange indicator the complete neutralization of HCO3 takes place. Indicates that only CO32 ions are present.
The alkalinity of water is the capacity of that water to accept protons. This alkalinity is easily. It is determined by titration with a standard solution of a strong mineral acid to the successive bicarbonate and carbonic acid equivalence points indicated electrometrically or by.
The alkalinity of water is the capacity of solutes to act as a base by reacting with protons. Alkalinity is usually imparted by bicarbonate carbonate and hydroxide. The absorbance is measured at 430 nm.

Late Oligocene To Early Miocene Humidity Change Recorded In Terrestrial Sequences In The Ili Basin South Eastern Kazakhstan Central Asia Hellwig 2018 Sedimentology Wiley Online Library

Microbialites And Trace Fossils From A Middle Triassic Restricted Carbonate Ramp In The Catalan Basin Spain Evaluating Environmental And Evolutionary Controls In An Epicontinental Setting Mercedes Martin 2021 Lethaia Wiley Online Library

Protein Function Biochemistry Chemistry Macromolecules

A Bei Image Of Paragenetic Sequence Of Cements In Quartz Q Siltstone Download Scientific Diagram

Doc316 52 93108 Langelier Index In Water
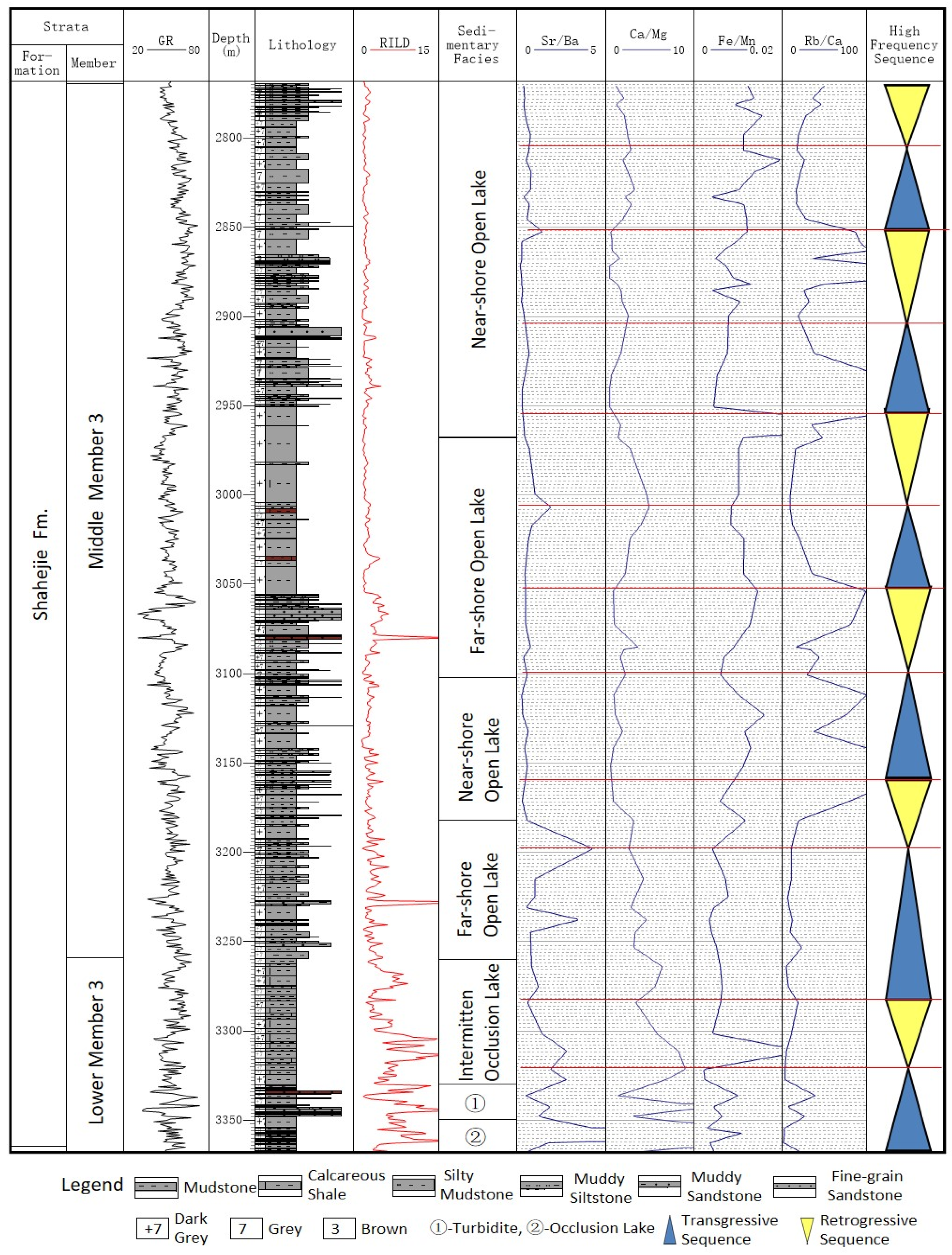
Minerals Free Full Text Application Of Elemental Geochemistry In High Frequency Sequence Stratigraphic Analysis Of Lacustrine Shale Html
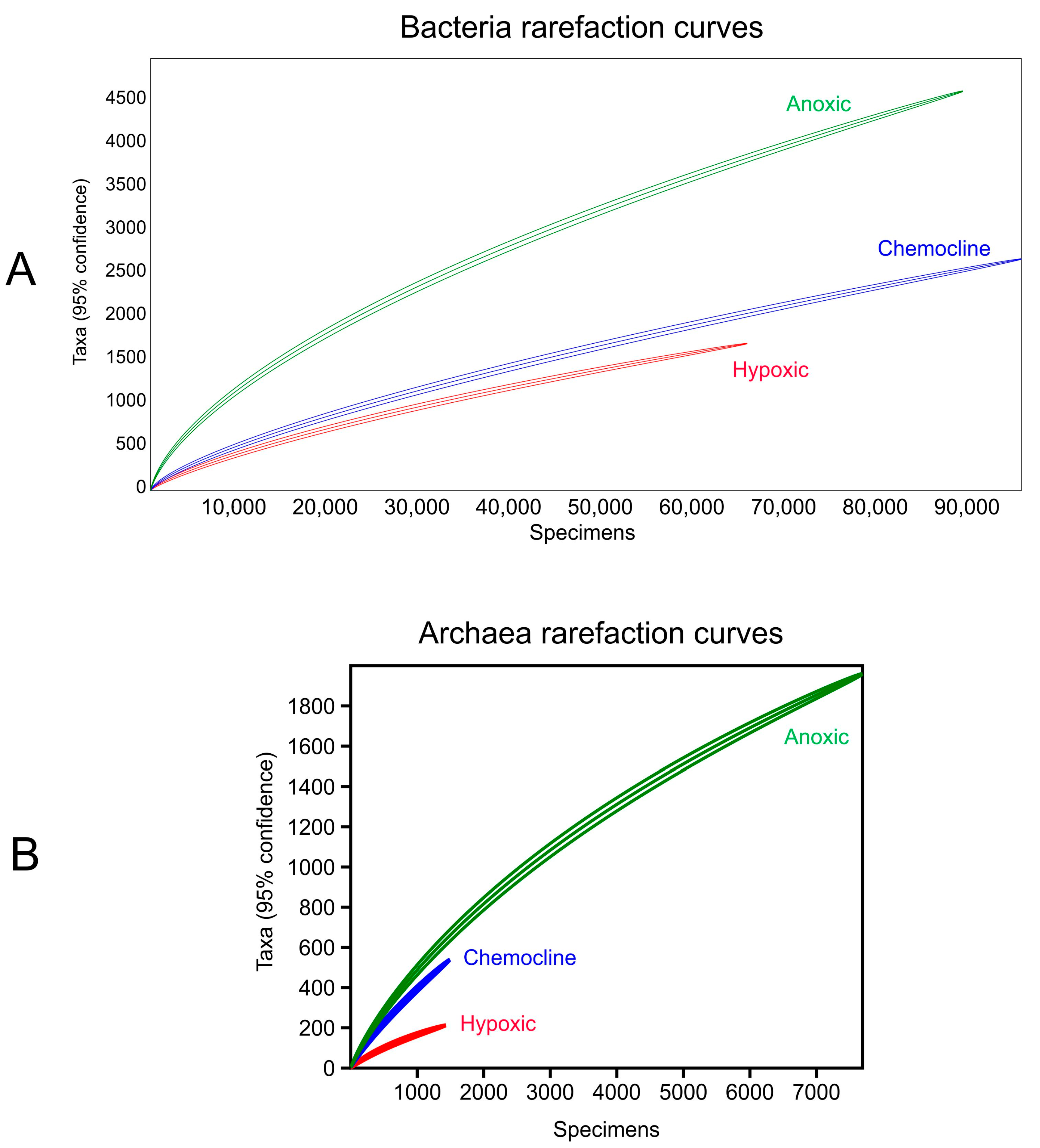
Water Free Full Text Microbial Function And Hydrochemistry Within A Stratified Anchialine Sinkhole A Window Into Coastal Aquifer Interactions Html

Pdf Sedimentary Facies Associations And Sequence Stratigraphy Of Source And Reservoir Rocks Of The Lacustrine Eocene Niubao Formation Lunpola Basin Central Tibet

Mid Cretaceous Lithostratigraphy And Sequence Stratigraphy For Qatar Download Scientific Diagram

Mid Cretaceous Lithostratigraphy And Sequence Stratigraphy For Qatar Download Scientific Diagram
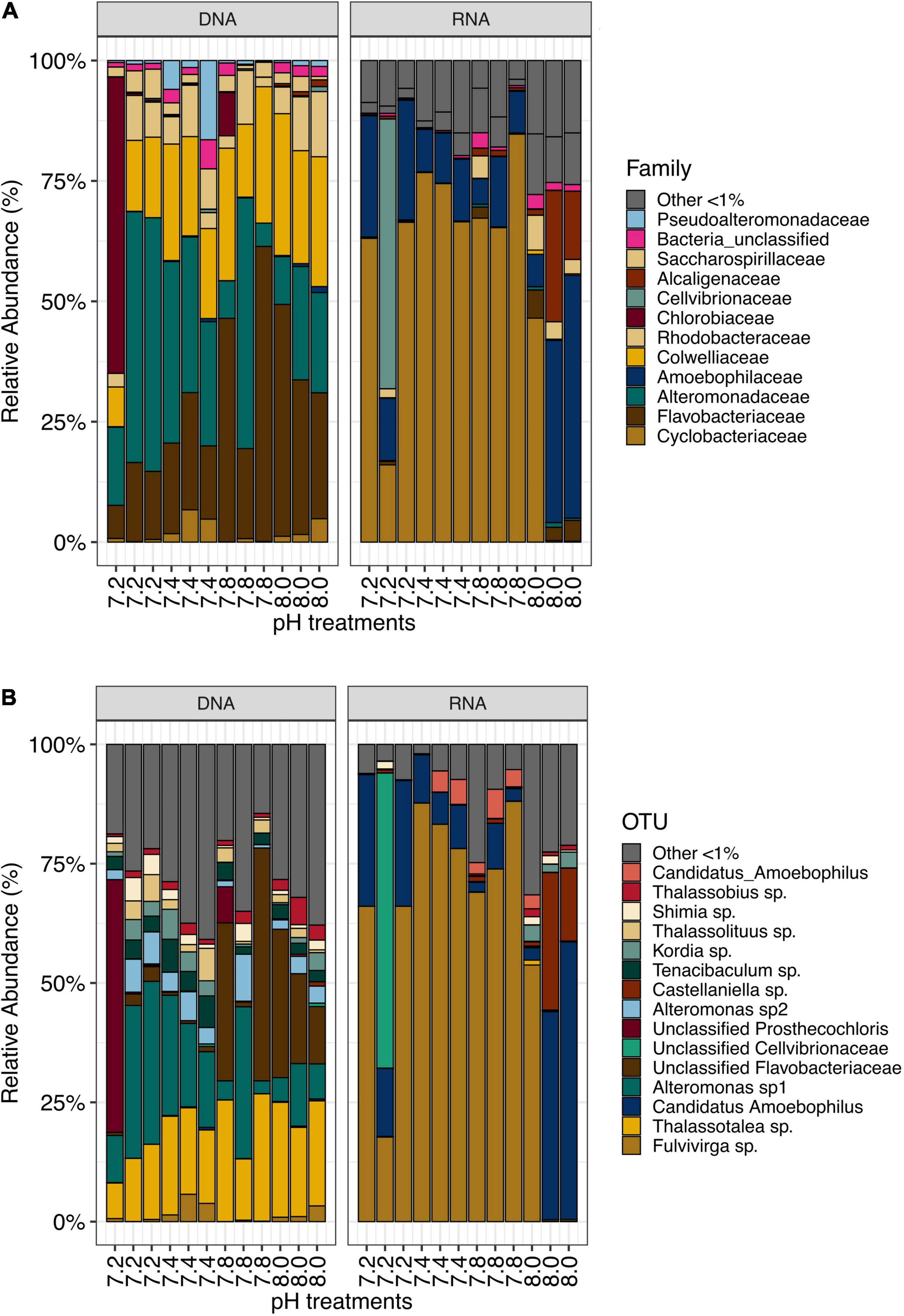
Frontiers Effects Of Ocean Acidification On Resident And Active Microbial Communities Of Stylophora Pistillata Microbiology

Stratigraphic Column Of The V1 Sequence Along Wadi Fizh Broken Lines Download Scientific Diagram

Comparison Of Chronostratigraphic Time Lines And Seismic Sequence Download Scientific Diagram
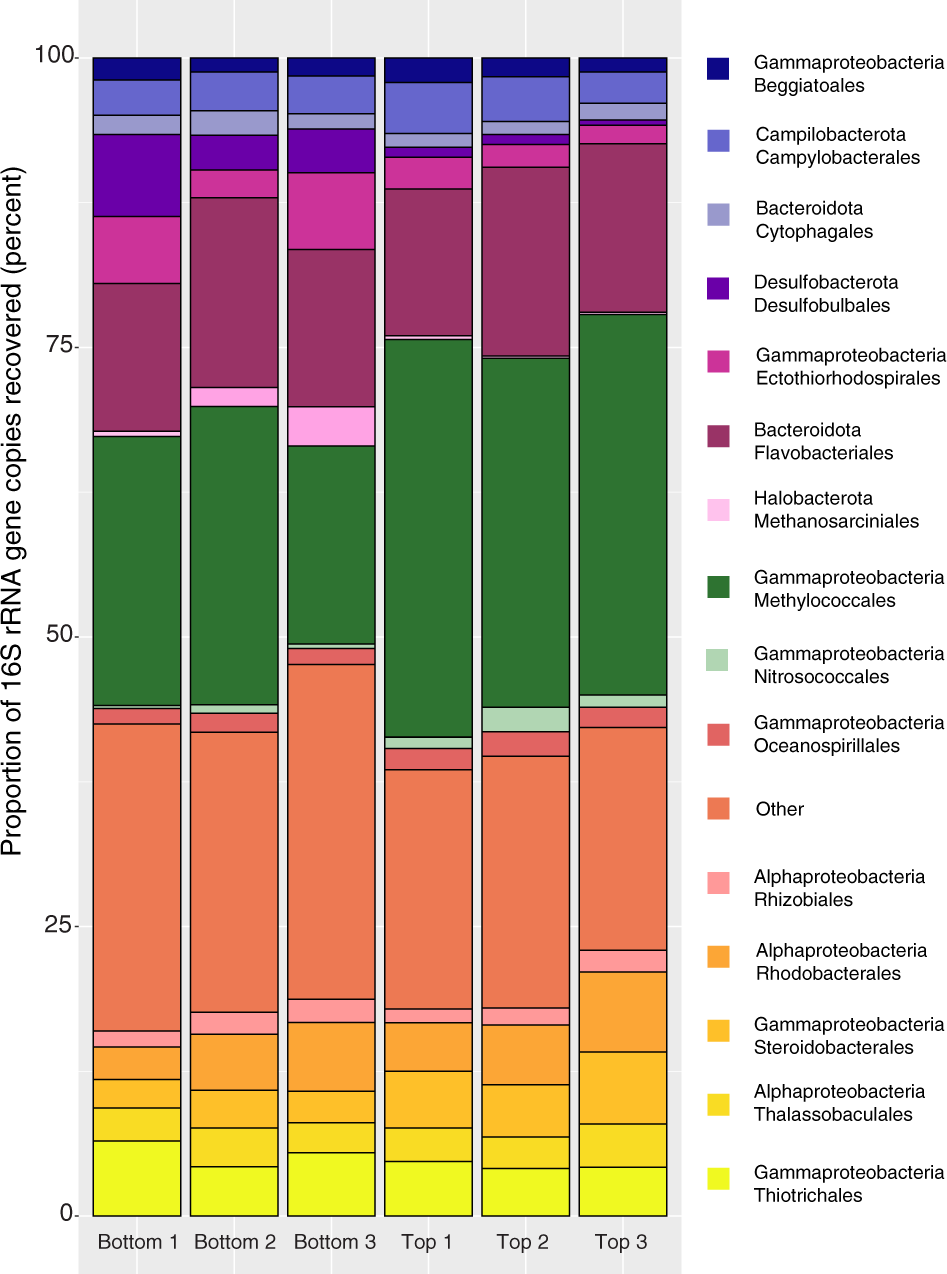
Sulfur Bacteria Promote Dissolution Of Authigenic Carbonates At Marine Methane Seeps The Isme Journal
Posting Komentar untuk "Carbonate Alkalinity Of Water Is Determined By Using Indicators In The Sequence"