Carbonate Bond Angle
The CO32- ion therefore has a trigonal-planar shape just like BF3 with a 120 degree bond angle. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-.
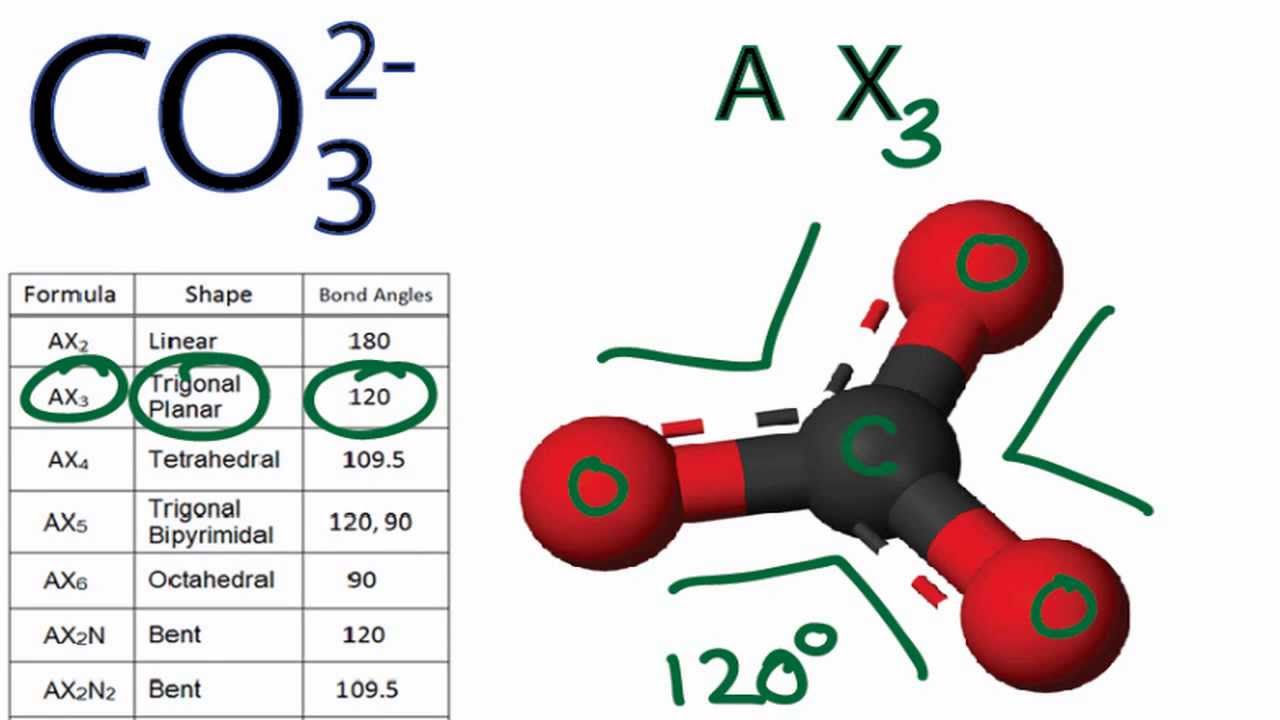
Co3 2 Molecular Geometry Shape And Bond Angles Youtube
3 By treating a solution of potassium hydroxide with carbon dioxide to produce potassium bicarbonate.

Carbonate bond angle. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. The hybridization of bromine must be sp3. Of the following species A.
Theoretically it should be 120 degrees but in real life is it actually exactly 120 degrees. The two C-O single bonds and the CO double bond. What is the hybridization of bromine in bro2.
A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. A compound with only 2 atoms would not have a. The H-C-H bond angle is 1176 while the H-C-C angle is 121.
3 that the molecular geometry of CO 3 2 is trigonal planar with bond angles of 120. What is the exact bond angle of the carbonate ion. SF6 Molecular Geometry Lewis Structure Shape and Polarity.
Using the example above we would add that H 2 O has a bond angle of 1095 and CO 2 would have a bond angle of 180. For example water should have bond angles of 1095 degrees but in reality its around 1045 or something like that. It is primarily used to show the relative positions of the different.
A bond angle is the angle between any two bonds that include a common atom usually measured in degrees. 1095 Trigonal geometry C120 D. It is a conjugate base of a hydrogencarbonate.
The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. A Lewis structure or Lewis representation also known as electron raster diagram Lewis raster formula Lewis point structure or point electron structure is a two-dimensional diagram used in chemistry to show the bonding between atoms of a molecule and the lone electron pairs that may be present in this molecule. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among.
Is produced by the following methods of manufacture. 60 will have bond angles of 109 2. The geometric shape of a tetrahedral molecule is created by its central atom bonding with four other atoms that surround it creating angels of 190 degrees Fahrenheit.
It is non-flammable odourless and colourless and is an excellent insulator. Carbonate ion CO 3 2-is trigonal planar in shape with a O-C-O bond angle of 120 o because of three groups of bonding electrons and no lone pairs of electrons. But these electrons are concentrated in three places.
Sulfur hexafluoride or SF6 is an inorganic greenhouse gas. But these electrons are concentrated on the 2 single C-O bonds and the CO double bond. Molecule bond VSEPR- WebMO Do both models Experimentally- angle of interest predicted calculated predict the same determined bond angle bond angle angle within 392 bond angles CH3NH2C-N-H 111 HCONH.
In the case of carbonate then there are three sigma bonds and no lone pairs so lone sigma 3 the arrangement of electron pairs is trigonal and the bond angles are all 120. 2 By treating a solution of potassium hydroxide with excess carbon dioxide to produce potassium carbonate. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom.
What is the equivalent Lewis structure for CO32. For CO 3 2-ion Total pairs of electrons are 12. What is the bond angle in the carbonate ion CO_3-2.
The C-H bond is sp 2 - s sigma with bond length 108 pm. The shape is deduced below using dot and cross diagrams and VSEPR theory and illustrated below. How scientists got that number was through experiments but we dont need to know too much detail because that is not described in the textbook or lecture.
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. Carbonate Ion is a polyatomic ion with formula of CO3 2-. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
HCONH0-C-N Same as 1247 resonance HCONH C. To be the center atom ability of having higher valance is important. 1 By electrolysis of potassium chloride followed by exposing the resultant potassium to carbon dioxide.
Electron repulsion is minimised when the 3 oxygen atoms. Therefore tetrahedrals have a bond angle of 1095 degrees. HCONH20-C-N 12470 resonance HCONH C-NH 120 and 11859 structure 1 HCONH.
CO3 2- Molecular Geometry Shape and Bond Angles - YouTube. Center atom of CO 3 2-ion. Chemistry questions and answers.
The VSEPR theory therefore predicts that CO 2 will be a linear molecule just like BeF 2 with a bond angle of 180 o. Total electron pairs are determined by dividing the number total valence electrons by two. The O-C-O bond angle in the CO32-ion is approximately A.
Carbonates are readily decomposed by acids. The formation of sigma and pi bon. Sodium carbonate contains two ionic bonds between one Na and CO3 and the other Na and CO3 because in water it brakes down to 2Na and CO3- ions.

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Is Bcl3 Polar Or Non Polar Boron Trichloride In 2021 Molecules Polar Boron

Ch2f2 Molecular Geometry Bond Angles Electron Geometry Difluoromethane In 2021 Molecular Geometry Covalent Bonding Molecular

Ch2o Molecular Geometry Shape And Bond Angles Formaldehyde In 2021 Molecular Geometry Molecular Geometry Shape

Is H2o Polar Or Nonpolar Water In 2021 Polarity Of Water Polar Molecules

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Boron

Hybridization Of Ch3oh Methanol In 2021 Chemistry Notes Education Online Classes

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Sf2 Molecular Geometry Bond Angles Electron Geometry Sulfur Difluoride In 2021 Molecular Geometry Geometry Molecular

Co32 Lewis Structure Carbonate Ion In 2021 Molecules Lewis Chemical Formula

Chf3 Lewis Structure Fluoroform In 2021 Molecules How To Find Out Lewis

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion In 2021 Molecular Geometry Molecular Geometry
Posting Komentar untuk "Carbonate Bond Angle"