Carbonate And Bicarbonate
Potassium will give purple colour to flame test. Bicarbonate is excreted and reabsorbed by your kidneys.

The Ultimate Guide On Food Grade Potassium Carbonate E Ingredients Com Carbonate Potassium Bicarbonate Food Additives
Sodium bicarbonate is also used as a odor neutralizer cleaning or exfoliating agent and sometimes as a temporary fire extinguisher.

Carbonate and bicarbonate. Separate the precipitate from the solution. Carbonates and bicarbonates are two groups of accelerators which can be used in sprayed concrete. When you observe a difference between total CO2 and bicarbonate that is larger than 5 the patient will be acidic.
The normal ratio of bicarbonate to carbonic acid at normal pH is around 201. Your blood brings bicarbonate to your lungs and then it is exhaled as carbon dioxide. Sodium carbonate is used for body processes or reactions.
Bicarbonate and carbonate are converted to carbon dioxide by lowering the pH of the solution then the absorbance of the dissolved carbon dioxide at 2345 wavenumbers is measured using a liquid sample cell. I think the bi- originated from the fact that the bicarbonate contains about twice as much carbonate releases t. Implicit in this statement is the relationship among dissolved carbonate species whether or not they are in equilibrium with solid phase metal carbonates.
Carbonic acid then decomposes into carbon dioxide and water explain Drs. The double value of titrant is the epm of carbonate if sample is taken 20 ml. HC03- H H2CO3 C032- 2H H2CO3 A separate aliquot of unknown is treated with excess standard NaOH to convert HCO3- to CO32-.
Hydrogencarbonate is an intermediate form in the deprotonation of carbonic acid. The results show that both of them could accelerate the. Additional acid to reduce pH 83 to 45 neutralizes remaining ½ carbonate already converted to bicarbonate and the bicarbonate -- solution turns orange a carbonate alkalinity is present when the phenolphthalein alkalinity is not zero but less than the total alkalinity.
Bicarbonate serves a crucial biochemical role. The key difference between calcium carbonate and calcium bicarbonate is that the calcium carbonate molecule consists of Ca C and O chemical elements whereas calcium bicarbonate consists of Ca C O and H chemical elements. Carbonate and Bicarbonate The carbon dioxide that is dissolved by naturally circulating waters appears in chemical analysis principally as bicarbonate and carbonate ions.
Ba2 CO32- BaCO3 s The. This regulates your bodys pH or acid balance. The ability to resist changes in pH by neutralizing acids or bases is called buffering.
Your kidneys also help regulate bicarbonate. The carbonate ion can react with calcium ions Ca which are in excess in seawater to form calcium carbonate CaCO3 the material out of which the shells of. Calcium carbonate is a carbonate of calcium that has the chemical formula CaCO 3.
Add 9274 g of Sodium carbonate anhydrous to the solution. To test potassium ion you can do flame test. Add barium chloride BaCl 2 solution to carbonate ion solution.
Mary Campbell and Shawn Farrell in. For Bicarbonate add few drops of mixed. Sodium bicarbonate and sodium carbonate act similarly in food though sodium carbonate reacts much more strongly.
The free acid itself bicarbonate ion ceHCO3- first-stage ionized form and carbonate ion ceCO32 second-stage ionized form. Potassium carbonate is soluble in water and a colourles solution. Add 105 g of Sodium bicarbonate to the solution.
In this study the effects of the two accelerators sodium carbonate Na 2 CO 3 and sodium bicarbonate NaHCO 3 0 1 2 3 and 4 by weight of ordinary Portland cement OPC on the properties of OPC paste were compared. That scheme works - sort of - and is found in lots of older books on carbonate analysis including Standard Methods but is approximate. Carbonate that follows this path represents a linkage between the carbon cycle and the hydrologic cycle.
In inorganic chemistry bicarbonate IUPAC-recommended nomenclature. Carbonate is the CO₃² ion. The bicarbonate and carbonate ions are responsible for the buffering capacity of seawater ie.
First total alkalinity moles of bicarbonate moles of carbonate is measured by titrating the mixture with standard HCI to a methyl orange end point. Total CO2 will therefore be about 5 higher than serum bicarbonate. Bicarbonate is the HCO₃ ion also called the hydrogen carbonate ion.
HC03- OH- CO32- H2O Then all the carbonate is precipitated with BaCl2. To use it one assumes that the bicarbonate content as CaCO3 is 2P -2OH- where P is the phenolpthalein alkalinity and OH- is as CaCO3 and that the bicarbonate content is T - 2P OH-. Seawater can resist drastic pH changes even after the addition of weak bases and acids.
Sodium carbonate is commonly used in neutralizing acidic solutions in various fields. However ecient transfer takes place only into the topmost 100 m wind-mixed layer. Bicarbonate also known as HCO3 is a byproduct of your bodys metabolism.
Both react with acid resulting in the production of a compound called carbonic acid which has the chemical formula H2CO3. Section 5- Carbonate Chemistry CARBONATE EQUILIBRIA Carbonates are arguably the most important dissolved component of soil solutions and in alkaline soils this statement is even less disputable. How to test for carbonate in water.
Thus sodium carbonate is Na₂CO₃ and sodium bicarbonate is NaHCO₃. Prepare 800 mL of distilled water in a suitable container. Sodium bicarbonate is a weaker base and is usually monoprotic.
Carbonate actually converted to bicarbonate by pH 7 b. Barium carbonate BaCO 3 white precipitate is given. What does bicarbonate mean.
Carbonate ions are able to react with and neutralize 2 hydrogen ions H and the bicarbonate ions are able to neutralize H or hydroxide ions OH- present in water. It occurs naturally and appears as a white solid. Carbonic acid ceH2CO3 has two ionizable hydrogens so it may assume three forms.
Of its transformation into bicarbonate and carbonate in a slightly alkaline aqueous medium and they contain about 60 times as much inorganic carbon as is in the atmosphere. Sodium bicarbonate is found in our body and is an important element. It is a polyatomic anion with the chemical formula H C O 3.
Answer 1 of 4. Add distilled water until volume is. Multiply the double of titrant volume by 30 you will get ppm of carbonate.

Multimedia Forming A Precipitate Chapter 6 Lesson 3 Chemical Equation Middle School Chemistry Lesson

Sugar Snake Mel Chemistry Carbonate Simple Sugar Complex Sugars

Sodium Bicarbonate Iupac Name Sodium Hydrogen Carbonate Commonly Known As Baking Soda Is A Chemical Compo Chemistry Science Chemistry Sodium Bicarbonate

Twitter Ocean Acidification Shell Game Ocean Wise

Chinese Snow Brand Sodium Bicarbonate Food Grade Sbc Baking Soda Sodium Hydrogen Carbonate Manufacturing Sodium Bicarbonate Baking Soda

Carbonate Bicarbonate Buffer Ph 9 2 To 10 6 Preparation Guide And Recipe Recipe Can Be Automatically Scaled By Entering D Preparation Recipes Chemistry Labs
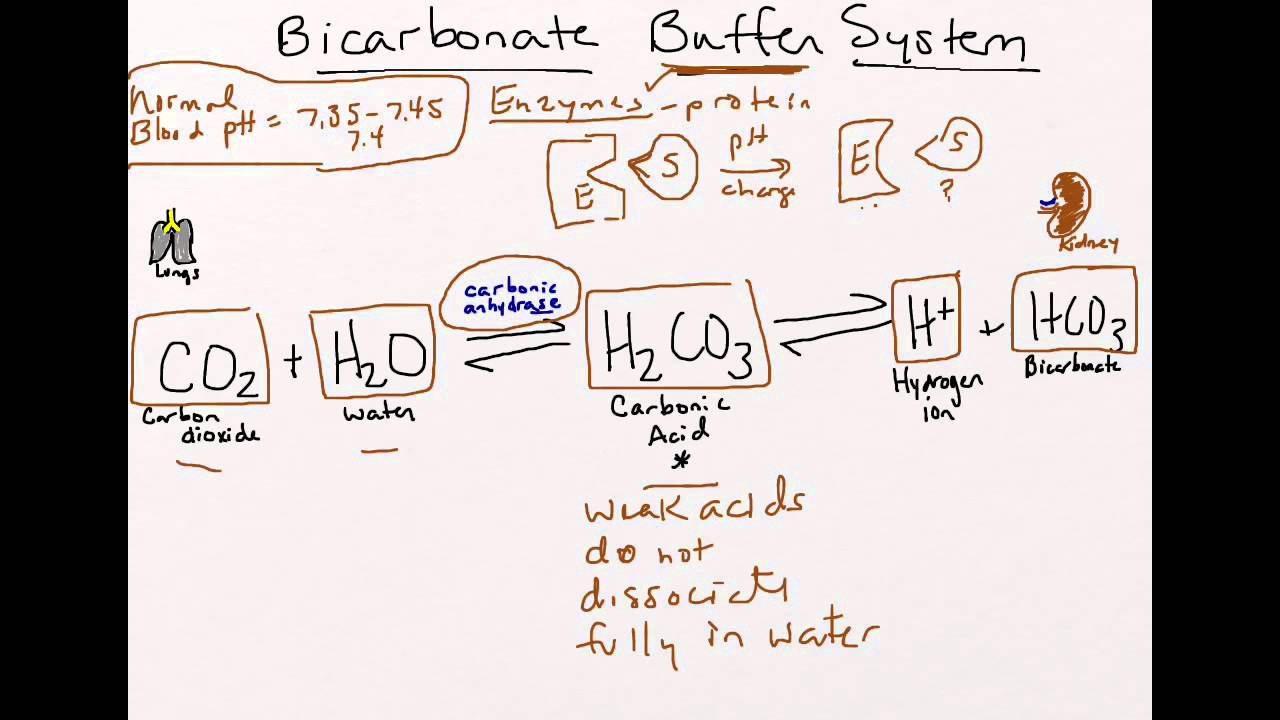
Bicarbonate Buffer System System Study Tips Pharmacy School

Image Result For Effects Of Ocean Acidification Ocean Acidification Carbonate Ocean Science

How To Make Washing Soda From Baking Soda Baking Soda Washing Soda Sodium Bicarbonate

Polyatomic Ion Chart 400 Bildung College Chemistry Teaching Chemistry Science Chemistry

The Compound S Scientific Name Is Sodium Hydrogen Carbonate But It Is Known By Many Other Names Around The World Some O Sodium Bicarbonate Baking Soda Baking



Posting Komentar untuk "Carbonate And Bicarbonate"