Carbonate Base Pka
Carbonate pK a 476 acetate carboxylate pKa Values of Common Bases Values in H 2O as much as possible so common comparisons ie H 2OpK a 157 can still be used Note. Some of the considered bases guanidino phosphorus carbenes are expected to reach gas-phase basicity around 370 kcal mol-1 thus being the most basic neutral bases ever reported.

Organic Bases Structures A The Effect Of Organic Bases On The Yield Download Scientific Diagram
Table of Acids with Ka and pKa Values CLAS Acid HA A-Ka pKa Acid Strength Conjugate Base Strength Hydroiodic HI I-Hydrobromic HBr Br-Perchloric HClO4 ClO4-Hydrochloric HCl Cl-Chloric HClO3 ClO3-Sulfuric 1 H2SO4 HSO4-Nitric HNO3 NO3-Strong acids completely dissociate in aq solution Ka 1 pKa 1.

Carbonate base pka. For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system. PKb logKb. At 25C pK_a pK_b 1400.
Nitric Acid - HNO. Soda ash also known as sodium carbonate Na2CO3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate-bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash. Under natural conditions H and OH are negligibly small compared to the carbonate species concentrations.
Bicarbonate has a pKa of 61 which is NOT ideal in normal physiologic conditions. Smaller values of pK_a correspond to larger acid ionization constants and hence stronger acids. AC HCO3 2CO 3 2 b 2c 915.
At each temperature there is a partial pressure of carbon dioxide that is in equilibrium with calcium carbonate. Additionally is ammonium carbonate acidic or basic. The acidity pKa value of sodium carbonate is 1033.
Bicarbonate is better described as a CO2 transport mechanism and not as a buffer protons combine with hydrogen ions which are at equilibrium with carbonic acid H 2. Conversely smaller values of pK_b correspond to larger base ionization constants and hence stronger bases. Ammonium carbonate is a very weakly acidic compound based on its pKa.
Potassium Chloride KCl 11-18. Sulfuric acid is the strongest acid on our list with a pK a value of 10 so HSO 4-is the weakest conjugate base. 1 HA H A-HA acid.
A- anion conjugate base 2 K H A - HA K thermodynamic equilibrium K equilibrium constant 3 C T HA A. The pKa for the second dissociation of the carbonic acidbicarbonatecarbonate system is about 1025 or maybe its 1023 but anyway assuming it is 1025 an equimolar mixture of carbonate and bicarbonate should give a pH of about 1025. At room temperature the equilibrium overwhelmingly favors calcium carbonate because the equilibrium CO 2 pressure is only a tiny fraction of the partial CO 2 pressure in air which is about 0035 kPa.
If the Cs2CO3 is in water then I would expect that the pKas for the base. The stronger the conjugate acid the weaker the conjugate base. Kb BHOH B.
Buffer pKa and pH Range Values For preparation of. The pK a values associated with bases is normally meant to refer to the true pK as of their conjugate acids. Carbonates are readily decomposed by acids.
Therefore when the salt is completely dissociated in an. In short the stronger the acid the smaller the pKa value and strong acids have weak conjugate bases. You can see that hydroxide ion is a stronger base than ammonia NH 3 because ammonium NH 4 pK a 92 is a stronger acid than water pK a 140.
CaCO 3-CO 2-H 2 O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. Of an acid and its conjugate anion base Ba as a function of pH pH -log H requires knowledge of the equation describing the acidbase equilibrium hydrogen ion exchange the apparent equilibrium constant K and information about the total concentration BaT of the acid in solution HBa H Ba IV-1.
Perchloric Acid HClO. Secondly is NH4Cl an acid or base. If it is under aqueous conditions then you need the pKa of H2CO3 and HCO3-.
Buffers in the pH. It is a conjugate base of a hydrogencarbonate. PKa Chart conjugate acid conjugate base conjugate acid conjugate base s t r o n g e s t a c i d s w e a k e s t b a s e s hydrogen sulfide 1 2 bicarbonate hydrochloric acid -7 carbocations -3.
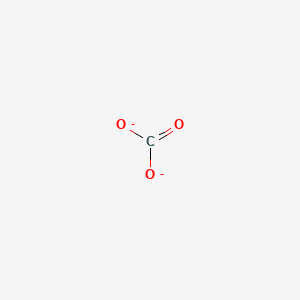
For most weak acids pKa ranges from 2 to 13. Carbonate Ion is a polyatomic ion with formula of CO3 2-. The answer to that question is obviously resonance structure of the carboxylic acid that stabilises the negative charge.
H hydrogen ion. The smaller the value of pKb the stronger the base. The classical substituted alkylphosphazenes were predicted to reach p K a values of 50 in acetonitrile.
The value of the equilibrium constant is given by. Oxalic Acid C. For most weak acids Kb ranges from 102 to 1013.
This is often sloppily used by. Ie pK a associated with HO-is 157 which is the pK a of H 2O. As mentioned in the other answer NH4Cl is an acidic salt formed by the neutralization of a strong acid HCl with a weak base NH3.
The sum of the weak acid and alkali-ion concentrations determined by an acid titration referred to as the total alkalinity thus about equals the carbonate alkalinity defined as. Predicting pH of an acidic salt of an weak acid and weak base. PKa values describe the point where the acid is 50 dissociated ie.
B H₂O BH OH. The answer to that question is to reverse it why does the carboxylic acid in the carbonate at a pka of 36 which is ballpark carboxylic acid. It is safer to handle thats why it is preferred over sodium hydroxide in various chemical processes.
Sodium carbonate is one of the common compounds we used in our home for purposes like washing clothes also known as washing soda or soda ash. Below are tables that include determined pKa values for various acids as determined in water DMSO and in the gas Phase. Buffers pKa range.
Hydrochloric Acid - HCl 0-2. Acidbase reactions always proceed in the direction that produces the weaker acidbase pair. In fact the pH range of effectiveness is probably 51 71 for the bicarbonate buffer system.
The greater the value of Kb the stronger the base. Name formula pK bn. NCI Thesaurus NCIt Carbonate is a carbon oxoanion.
-water system unless other sources of base are added.

Flinnprep Courses Chemistry Lessons Teaching Chemistry Chemistry Classroom

Naming Ions And Ionic Compounds Chemistry Lessons Science Chemistry Chemistry Education

Pin By Kelly Reed On Chemistry Science Chemistry High School Chemistry Chemical Science

Common Polyatomic Ions Chemistry Lessons Teaching Chemistry Chemistry Education
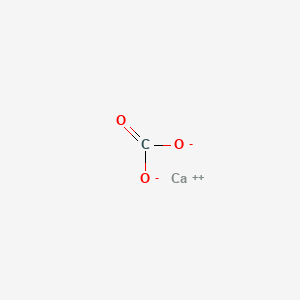
Calcium Carbonate Caco3 Pubchem

Free Energy Profiles For Carbonate Formation With Bromine As Catalyst Download Scientific Diagram

Scheme 2 General Reaction Mechanism Of Cyclic Carbonate Formation In Download Scientific Diagram

Acids And Bases Teaching Chemistry Chemistry Review Chemistry
Sodium Carbonate Na2co3 Pubchem

Ethyl Formate Ethyl Chloroformate And Diethyl Carbonate In Crossed Claisen With Ketones Organic Chemistry Organic Chemistry Study Chemistry Lessons

Compositions Of Hco3 Co3 2 Carbamate And Carbonate Before And After Download Scientific Diagram

Functional Groups In Organic Chemistry With Diagrams Organic Chemistry Functional Group Organic Chemistry Study

Posting Komentar untuk "Carbonate Base Pka"