Carbonate Buffer System Equation
The bicarbonate buffering system equation When CO 2 dissolves in water it reacts with an H 2 O molecule forming carbonic acid. Other mechanisms that assist in this function include the hemoglobin molecule in your red blood cells which also helps to buffer blood pH.
The above equation for K a can be rearranged to solve.
Carbonate buffer system equation. After carbon dioxide is dissolved it combines with the water molecules to form. Carbonate-Bicarbonate Buffer pH 92 to 106 preparation guide and recipe. The carbonic acid - bicarbonate buffer system consists of carbonic acid a weak acid and the bicarbonate anion its conjugate base.
The CarbonateBicarbonate Buffer System. The relationship between the components in the oceans buffer system can be represented by the equation below. Carbonic acid dissociates into bicarbonate and carbonate according to the following equations.
NIWA 2016 states that this natural buffering ability that the ocean possesses is called a carbonate buffer system. Two important biological buffer systems are the dihydrogen phosphate system and the carbonic acid system. The most important examples of biological buffer systems are as follows.
This equation relates the pH the ionization constant of a weak acid and the concentrations of the weak acid and its salt in a buffered solution. To log in and use all the features of. Remember that at a pH lower than the system point HA A- at a pH higher than the system point A-HA.
Carbonate buffers Theory A classic buffer is a combination of a weak acid and its conjugate salt. Well in any buffer system the boost in. However the relationship shown in Equation 11 is frequently referred to.
H 2 CO 3 H 2 O H 3 O HCO 3 -. This equation is applicable to all the buffer systems. Carbonate-bicarbonate buffer is used.
If youre seeing this message it means were having trouble loading external resources on our website. The important thing to realize here is that carbonic acid H 2CO3 is actually formed when carbon dioxide CO2 is dissolved in water. 3 Locate system point pH pK and HA A-.
Calcium carbonate CaCO3 is a very common mineral. CO2 atmos CO2aqH2O H2CO3 HHCO3-. Carbonic acid is diprotic which means in has two H ions to donate to solution.
This is the general equilibrium equation that describes the carbonic acid bicarbonate system. 2 3 -3 o - 64 H CO 2 3 H HCO K 10 H CO 3 where H 2 CO 3 H 2 CO 3 CO 2 aq H 2 CO 3 Note that Ko H2CO3 210 -4 or pK 369 if corrected for CO 2aq. This buffer system can be written as.
This produces a solution of bicarbonate. How does a carbonate buffer system work. Note that the cross over is 03 log units below the CT line for the acetic acid above this is at pH 47 4 Draw lines for the species slope 1 for A- and slope -1 for HA.
In this case CaCO3 calcium carbonate acts as a buffer in the bicarbonate form HCO3. Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings. PH pKa log A HA pH p K a log A HA where p Ka is the negative of the common logarithm of the ionization constant of the weak acid p Ka log Ka.
The pH of blood depends on the ratio of carbon dioxide to bicarbonate. For instance carbonic acid H 2CO 3 and sodium bicarbonate NaHCO 3 or even sodium bicarbonate and calcium carbonate. A buffer system exists to help neutralize the blood if excess hydrogen or hydroxide ions are produced.
The buffer capacity measured as the ability of the solution to minimize changes in pH due to addition of base is strongest near the midpoint of a titration when A HA and pH pK a. The bicarbonate buffer system is an effective buffer system despite having a low pKa because the body also controls pCO2. Equation 11 does not meet the strict definition of a Henderson-Hasselbach equation because this equation takes into account a non-acid-base reaction ie the dissociation of carbonic acid to carbon dioxide and water and the ratio in parentheses is not the concentration ratio of the acid to the conjugate base.
What happens when you titrate this combination with the strong acid of your choice. The phosphate buffer system operates in the internal fluid of all cells. One that is important in surface waters is the carbonic acidbicarbonate buffer.
In the human stomach and duodenum the bicarbonate buffer system serves to both neutralize gastric acid and stabilize the intracellular pH of epithelial cells via the secretion of bicarbonate ion into the gastric mucosa. H CO3 2- - HCO3-. Limestone is one familiar form of calcium carbonate.
An acid-base buffer consists of a weak acid H 2 CO and its conjugate base HCO 3. The role of the bicarbonate buffer system in regulating blood pH practice Khan Academy. Recipe can be automatically scaled by entering desired final volume.
421 p H p K a log 10 H C O 3 003 p C O 2. Carbonic acid H2CO3 a compound. What is the chemical equation for the carbonic acid buffer system.
CO 2 g H 2 O l H 2 CO 3 aq HCO 3 aq H aq. Carbonate System 1 3 MAR 510 Chemical Oceanography Carbonate Equilibrium Equations CO 2 g CO 2 aq CO 2aq H 2 O H 2 CO 3 H 2 CO 3 H HCO 3-HCO 3- H CO 3 2-Ca 2 CO 3 2- CaCO 3s calcite or aragonite. When significant amounts of both carbonic acid and bicarbonate are present a buffer is formed.
Carbon dioxide plays a vital role in the chemistry of sea water. In humans and other animals the carbonate buffering system helps maintain a constant pH in the bloodstream. So CaCO3 will first disassociate into Ca2 and CO3 2- you will then need to add some acid into the solution to change CO3 into HCO3.
A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate with acetic acid or ammonia with ammonium chloride. The chemical equation for this reversible reaction is. This buffer system consists of dihydrogen phosphate ions H 2 PO 4 as hydrogen ion donor acid and hydrogen phosphate ions HPO 2-4 as.
The Phosphate Buffer System. When the first proton is donated HCO3- otherwise known. When atmospheric carbon dioxide is dissolved in seawater carbonic acid H 2 CO 3 is formed.
Acids in acid rain promote the dissolution of calcium carbonate by reacting with the carbonate anion.

Blood Buffering Carbonic Acid Bicarbonate Youtube

Ocean Chemistry Acidification Time Scavengers
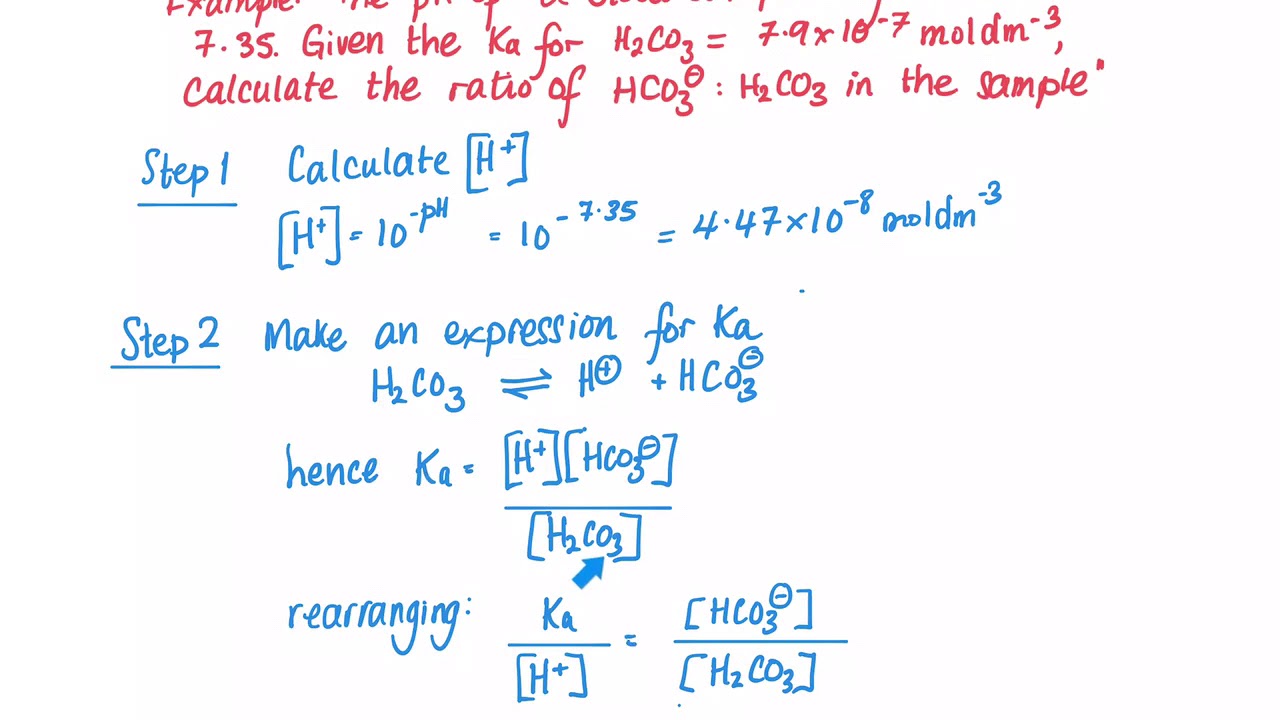
How To Calculate The Hco3 H2co3 Buffer Ratio In Blood Youtube

Ocean Chemistry Acidification Time Scavengers

Ocean Chemistry Acidification Time Scavengers

Ocean Chemistry Acidification Time Scavengers

Bicarbonate The Primary Buffer Youtube
How Buffers Help You Periodical 2015



Posting Komentar untuk "Carbonate Buffer System Equation"