Carbonate Bicarbonate Buffer System
Carbonate Buffered solution 01M Sodium Bicarbonate-Sodium Carbonate Buffer pH 90 UPR16490 250 ml - Applications. The pH of blood depends on the ratio of carbon dioxide to bicarbonate.

Chemistry Investigatory Project On Antacids Investigatory Project Chemistry Chemistry Projects
HCO3 and CO3 2.

Carbonate bicarbonate buffer system. Calcium carbonate CaCO3 is a very common mineral. What happens when you titrate this combination with the strong acid of your choice. CarbBicarb Buffer pH 92 Eye Protection.
Because of its high concentration in the extracellular fluid ECF bicarbonate acts as the dominant buffer of the ECF. A Brief Summary of Carbonate Buffer System Chemistry Atmospheric CO 2 dissolves in seawater and is hydrated to form carbonic acid H 2 CO 3. The CarbonateBicarbonate Buffer System.
Therefore at pH 85 you will have both carbonate and bicarbonate present. -water system unless other sources of base are added. When significant amounts of both carbonic acid and bicarbonate are present a buffer is formed.
Click to see full answer. For the case of a fixed partial pressure of carbon dioxide and calcium carbonate dissolved in the aqueous phase one more equation is need to describe the system. Any process which removes carbon dioxide as does photosynthesis tends to cause precipitation of calcium carbonate from solution especially where the bicarbonate.
They are in about a 101 ratio. Carbonic acid H2CO3 a compound. How does a carbonate buffer system work.
When the first proton is donated HCO3- otherwise known. In natural systems there are many buffers. This reaction then takes the aqueous carbon dioxide gas and dissolves it in the liquid water in order to combine and produce aqueous carbonic acid.
For some purposes you might want a weaker buffer. Recall that buffers are mixtures of weak acids and their conjugate bases that resist changes in pH. Importantly the body can independently modulate the concentrations of both the weak acid and weak base forms of the bicarbonate buffer.
One simple way to make your buffer is to make two 100mM solutions one of NaHCO 3 and one of Na 2 CO 3. After carbon dioxide is dissolved it combines with the water molecules to form. The role of the bicarbonate buffer system in regulating blood pH This is the currently selected item.
A buffer system exists to help neutralize the blood if excess hydrogen or hydroxide ions are produced. Buffer pH would decline as the pH decreases from neutrality. Carbonate buffers Theory A classic buffer is a combination of a weak acid and its conjugate salt.
The carbonic acid - bicarbonate buffer system consists of carbonic acid a weak acid and the bicarbonate anion its conjugate base. The bicarbonate buffering system is an crucial buffer system in the acid-base homeostasis of all living things. The bicarbonate buffer system is the main buffering system used in the body.
The important thing to realize here is that carbonic acid H 2CO3 is actually formed when carbon dioxide CO2 is dissolved in water. Important endogenous natural buffer systems include carbonic acidsodium bicarbonate and sodium phosphate in the plasma and hemoglobin and potassium phosphate in the cells. The Bicarbonate System The most prevalent ruminal buffer is HCO3 Counotte et al 1979.
Other mechanisms that assist in this function include the hemoglobin molecule in your red blood cells which also helps to buffer blood pH. Sodium Carbonate Anhydrous ACS grade 141321 1 Kg 141322 25kg 141321 MW. One that is important in surface waters is the carbonic acidbicarbonate buffer.
The carbonate buffer also reflects the ionic composition and the buffer capacity of small intestinal fluids. As a result the modulation of the bicarbonate buffer is used. Wear impervious protective clothing including boots gloves lab coat apron or coveralls as appropriate to prevent skin contact Protection of Hands.
While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. 497-19-8 Sodium Carbonate Anhydrous Reagent Grade 08499A 1 Kg - MW. When atmospheric carbon dioxide is dissolved in seawater carbonic acid H 2 CO 3 is formed.
CaCO 3-CO 2-H 2 O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide. Two important biological buffer systems are the dihydrogen phosphate system and the carbonic acid system. In the body a bicarbonate buffer system is used to regulate the pH of blood.
Well in any buffer system the boost in. The HCO3is of primary importance to buffering the blood of animals because it may be protonated to H2CO3. Carbonic acid is divalent.
That is it can undergo two de-protonation reactions to form bicarbonate HCO 3- and carbonate CO 3 2-. True False Increasing alveolar ventilation increases extracellular fluid H concentration and decreases pH. Using optical traps to manipulate single DNA strands.
4 Calcium bicarbonate in solution is a good buffer system and thus resists change in pH but it remains in solution only in the presence of a certain amount of free carbon dioxide. Coating protein oon microplates. What is the chemical equation for the carbonic acid buffer system.
In the human stomach and duodenum the bicarbonate buffer system serves to both neutralize gastric acid and stabilize the intracellular pH of epithelial cells via the secretion of bicarbonate ion into the gastric mucosa. Therefore this buffer system is essential for a realistic simulation of intestinal conditions. However as they play a minor role the bicarbonate buffer is considered being the most biorelevant buffer system for the simulation of intestinal conditions 78.
For instance carbonic acid H 2CO 3 and sodium bicarbonate NaHCO 3 or even sodium bicarbonate and calcium carbonate. Carbon dioxide plays a vital role in the chemistry of sea water. In humans and other animals the carbonate buffering system helps maintain a constant pH in the bloodstream.
Carbonic acid H 2 CO 3 is a weak acid and is therefore in equilibrium with bicarbonate HCO 3- in solution. Of course it all depends on what one wants to accomplish. This system relies on a dual equilibrium process where cellular respiration produces aqueous carbon dioxide gas and liquid water.
In the case of carbonatebicarbonate that would be an equi-molar ratio of carbonate and bicarbonate. Carbonic acid is diprotic which means in has two H ions to donate to solution. The main role of the bicarbonate system is to regulate and control the pH of blood and counteract any force that will alter the pH.
In physiology the body uses the carbonic acidbicarbonate system to adjust the pH of blood. Tightly sealed safety glasses or face shield. The bicar-bonate system includes two major ionic forms.

Pin On Fish Aquarium Water Treatments

Seachem Reef Buffer 8 8 Fl Oz Petco In 2021 Saltwater Tank Reef Aquarium Planted Aquarium

10 99 10 07 For Coral Propagation Coral Accel Should Be Used Daily It Is A Clean Product However Water Quality Should Always Be Soft Corals Marine Coral

Liquid Marine Buffer 20 L 5 3 Fl Gal Aquarium Water Treatments Marine Marine Aquarium

Hagen Rim Clamp For Aquaclear 201 301 Powerhead Rim Aquarium Supplies Clamp

Esv Aquatics Bionic 2 Part Calcium Buffer System For Aquarium Healthy Digestive System Healthy Fish Koi Fish Food
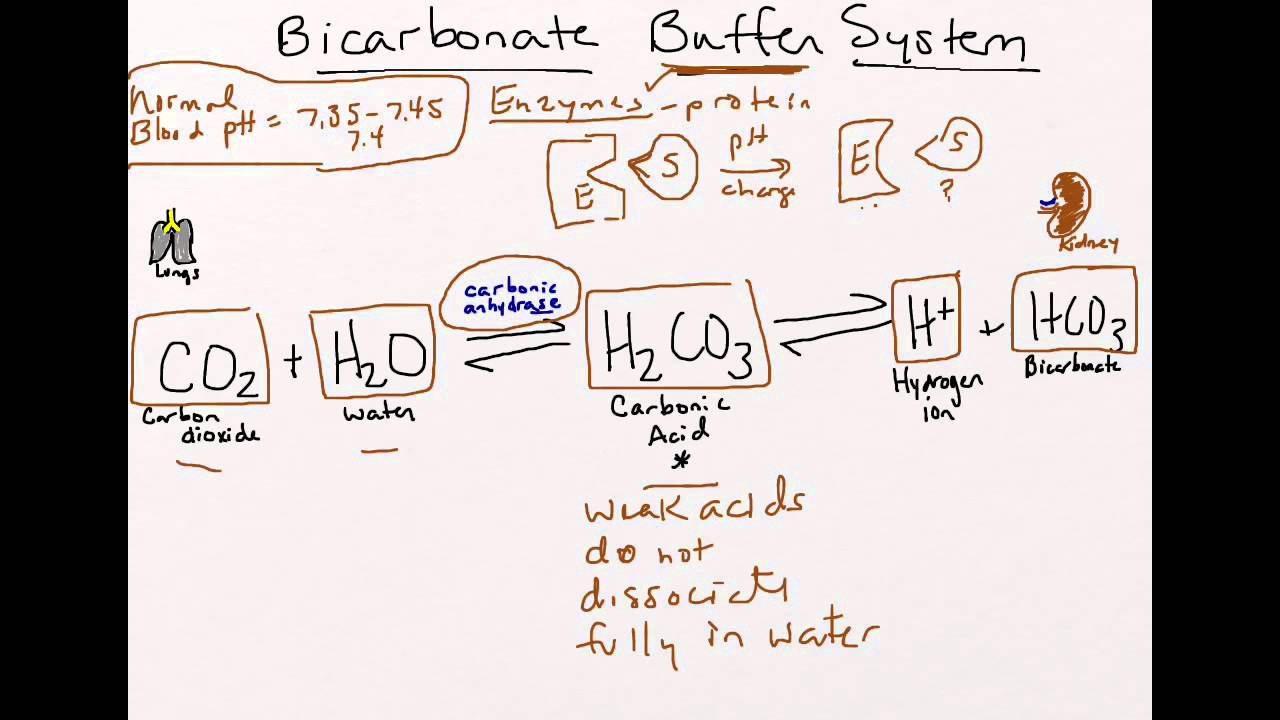
Bicarbonate Buffer System System Study Tips Pharmacy School

Kordon 31261 Amquel Ammonia Detoxifier For Aquarium 1 Gallon Gallon Aquarium Water Treatment Water Treatment

Tanganyika Buffer 4 Kg 8 8 Lbs Pet Supplements Fish Pet Koi Fish Food

Reef Calcium Is A Concentrated 50 000 Mg L Bioavailable Polygluconate Complexed Calcium Intended To Maintain Calcium In T Calcium Amazon Deals Shopping Reef

Aussie Symphyllia Wysiwyg Live Coral Ebay In 2021 Live Coral Underwater Creatures Coral



Posting Komentar untuk "Carbonate Bicarbonate Buffer System"