0.05 M Carbonate-bicarbonate
Ammonium bicarbonate appears as a white crystalline solid having the odor of ammoniaSoluble in waterThe primary hazard is the threat to the environment. Carbonate-Bicarbonate Buffer pH 92 to 106 preparation guide and recipe.
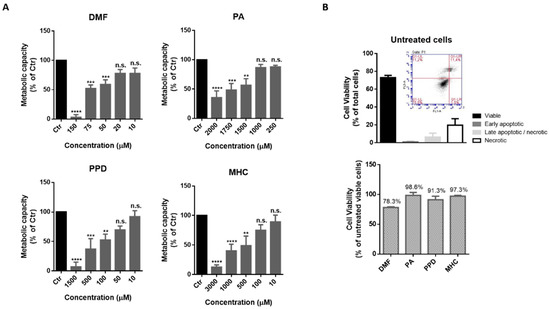
Ijms Free Full Text Calcium Modulation Anti Oxidant And Anti Inflammatory Effect Of Skin Allergens Targeting The Nrf2 Signaling Pathway In Alzheimer S Disease Cellular Models Html
This reaction results in a neutral salt.
0.05 m carbonate-bicarbonate. Protein coating on microtiter plates and plastic tubes. Upon dissociation sodium bicarbonate forms sodium and bicarbonate ions. What is the pH of nh4oh.
Of 005 M H2SO4 will be required to neutralize the total amount of carbonates. Sample water to bicarbonate phenolphthalein reading then 2X ml. 01 M Sodium carbonate.
The buffer is supplied as exactly pre-weighed tablets giving 100 ml of 005 M sodium carbonate-bicarbonate buffer with pH 96 at 25 C when dissolved in deionized water. The contents of one capsule yields 100 ml of 005 M carbonate-bicarbonate buffer pH 96 at 25. Each mL of 005 M edetate disodium consumed is equivalent to 4216 mg of MgCO 3.
The solution contained 005 M Na 2 CO 3 01M NaHCO 3 with a pH of 97. Ion formation increases plasma bicarbonate and buffers excess hydrogen ion concentration resulting in raised blood pH. For example magnesium carbonate pound for pound raises pH 17 times as much as calcium carbonate.
P264 - P280 - P305 P351 P338 - P337 P313. Prepare a 02-M solution of sodium bicarbonate 168 g100 mL. Molarity M 0025M Volume V 100mL 01L 1000mL 1L Let us say M₂ Molar mass of Na₂CO₃ and w mass of Na₂CO₃ Moles of Na₂CO₃ Mass of Na₂CO₃ Molar mass of Na₂CO₃ w M₂ M₂ 106 gmol Then Mol.
If you cannot find the answer to your problem below then please contact us or telephone. Coating Buffer 005 M Carbonate-Bicarbonate pH 96 Wash Solution 50 mM Tris 014 M NaCl 005 Tween 20 pH 80 Blocking Postcoat Solution 50 mM Tris 014 M NaCl 1 BSA pH 80 SampleConjugate Diluent 50 mM Tris 014 M NaCl 1 BSA 005 Tween 20 pH 80 Enzyme Substrate TMB Stop Solution 018 M H2SO4 or other appropriate solution. Molarity moles of solute volume of solution in L We have.
Sodium Bicarbonate is the monosodium salt of carbonic acid with alkalinizing and electrolyte replacement properties. Prepared buffer is suitable for use in protein coating procedures on multiwell plates or plastic tubes. Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings.
In addition Table 4 shows different corrosion parameters obtained from these curves. Is MgSO4 basic or acidic. Of 005 M H2SO4 is required to convert the quantity of carbonates present in 25 ml.
0050 M sodium Carbonate-Bicarbonate Buffer pH 96 at 25C. Three years after production date. Final pH will be 92.
Alkalinity in ground water arises mostly from bicarbonate ion formed when carbonic acid dissolves limestone. Of 005 M H2SO4 are required to. Low concentrations 005-01 of antimicrobial.
Assay for sodium bicarbonate Standard preparations Dissolve a suitable quantity of sodium chloride previously dried at 125 for 30 minutes and accurately weighed in water and dilute quantitatively with water to obtain a solution having a known. Recipe can be automatically scaled by entering desired final volume. 1 and Table 4 it can be seen that the critical and passive currents i critical and i.
Combine 4 mL of carbonate solution from Step 1 and 46 mL of bicarbonate solution from Step 2. 96 005 at 25C. Applications Protein coating on microtiter plates and plastic tubes.
The buffer is supplied in two formulations one of which contains 005 sodium azide as preservative. MW 1060 01 M Sodium bicarbonate. What is the pH of 005 m.
All solutions were prepared. 005 sodium azide as preservative. MgSO4 This salt was formed from the reaction of a strong base magnesium hydroxide with strong acid sulfuric acid.
Answer 1 of 2. Indicated and adjust the final volume to 200mL with deionized water. X mL of HCl is used when phenolphthalein is the indicator and y mL of HCl is used when methyl orange is the indicator in two separate titrations.
Figure 1 shows the polarization curves for the pipeline steel in 1 M bicarbonate-05 M carbonate 05 M bicarbonate-05 M carbonate and 005 M bicarbonate-05 M carbonate solutions. 01M sodium chloride was added to investigate the occurrence of pitting corrosion. The buffer is supplied as exactly pre-weighed tablets giving 100 ml of 005 M sodium carbonate-bicarbonate buffer with pH 96 at 25 C when dissolved in deionized water.
Adjust the final pH using 1 N HCl or 1 N NaOH. Previous Next Article Table of Contents. Protein coating on microtiter plates and plastic tubes for RIA and EIA.
Carbonate-bicarbonate buffer is used extensively in molecular and cell. Empty the contents of one capsule in 100 mL of deionized water and dissolve. Immediate steps should be taken to limit spread to the environment.
Bring to 200 mL with H 2 O. The contents of one capsule yields 100 ml of 005 M carbonate-bicarbonate buffer pH 96 at 25 C. Concentrated carbonatebicarbonate solutions were used to simulate the electrolytes trapped under permeable coatings such as asphalt on the buried pipelines.
Thus each mmole of bicarbonate arose when a half mmole of limestone was dissolved and 61 mg of bicarbonate 1 mmol is equivalent in this sense but only in this sense to 50 mg 12 mmol of limestone. CO2 H2O CaC03 -- Ca 2HCO3-. Click hereto get an answer to your question 40 ml of 005 M solution of sesqui - carbonate Na2CO3NaHCO3 2H2O is titrated against 005 M HCl.
84 gL MW 840 Mix sodium carbonate and sodium bicarbonate solutions in the proportions. The buffer is supplied as exactly pre-weighed tablets giving 100 ml of 005 M sodium carbonate-bicarbonate buffer with pH 96 at 25 C when dissolved in deionized water.
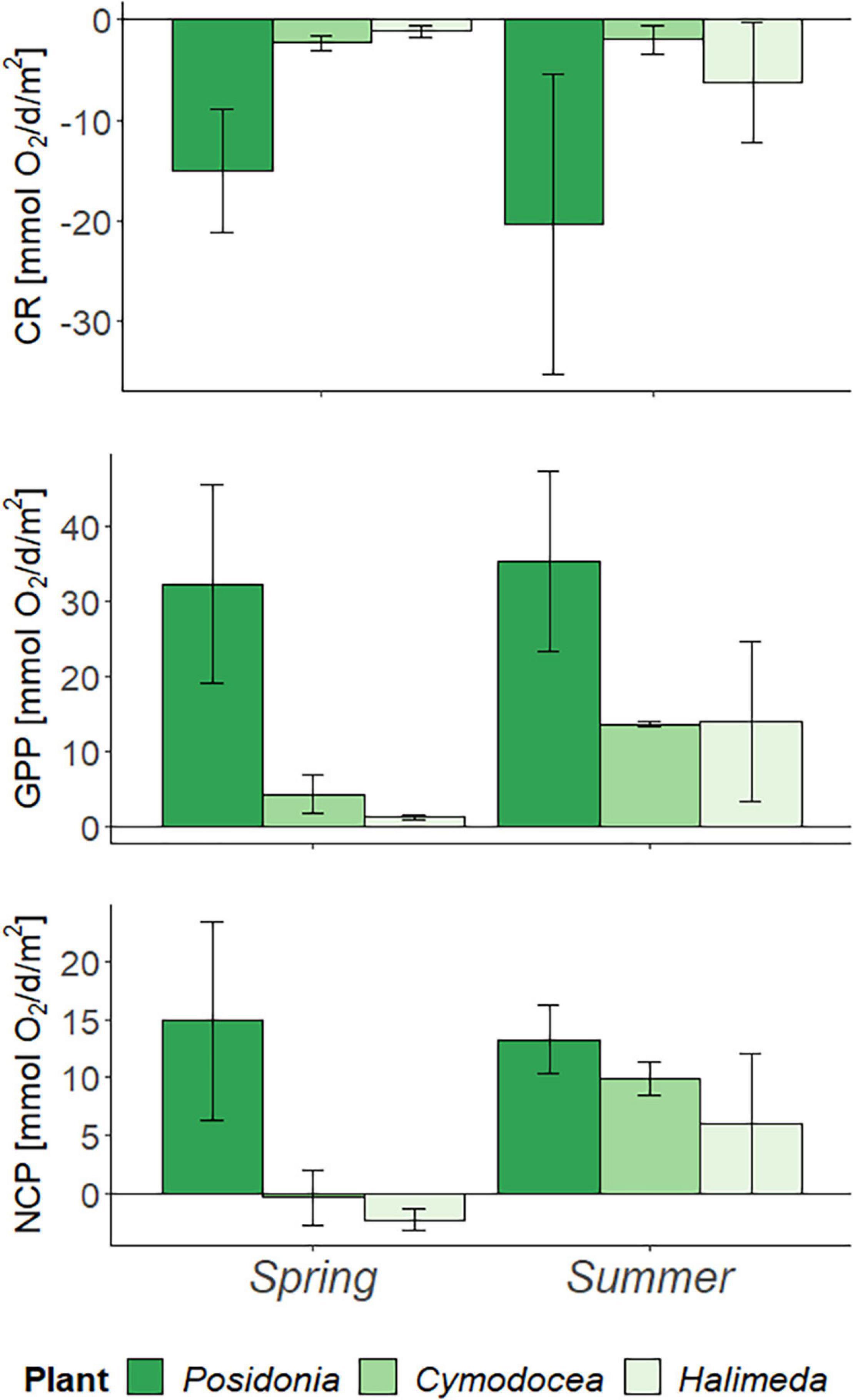
Frontiers Marine Macrophytes As Carbon Sinks Comparison Between Seagrasses And The Non Native Alga Halimeda Incrassata In The Western Mediterranean Mallorca Marine Science

How To Prepare Standardization 0 1 N Potassium Dichromate In 2021 Potassium Dichromate Starch Solution Potassium

What Is The Recipe To Make A Carbonate Bicarbonate Coating Buffer For Elisa
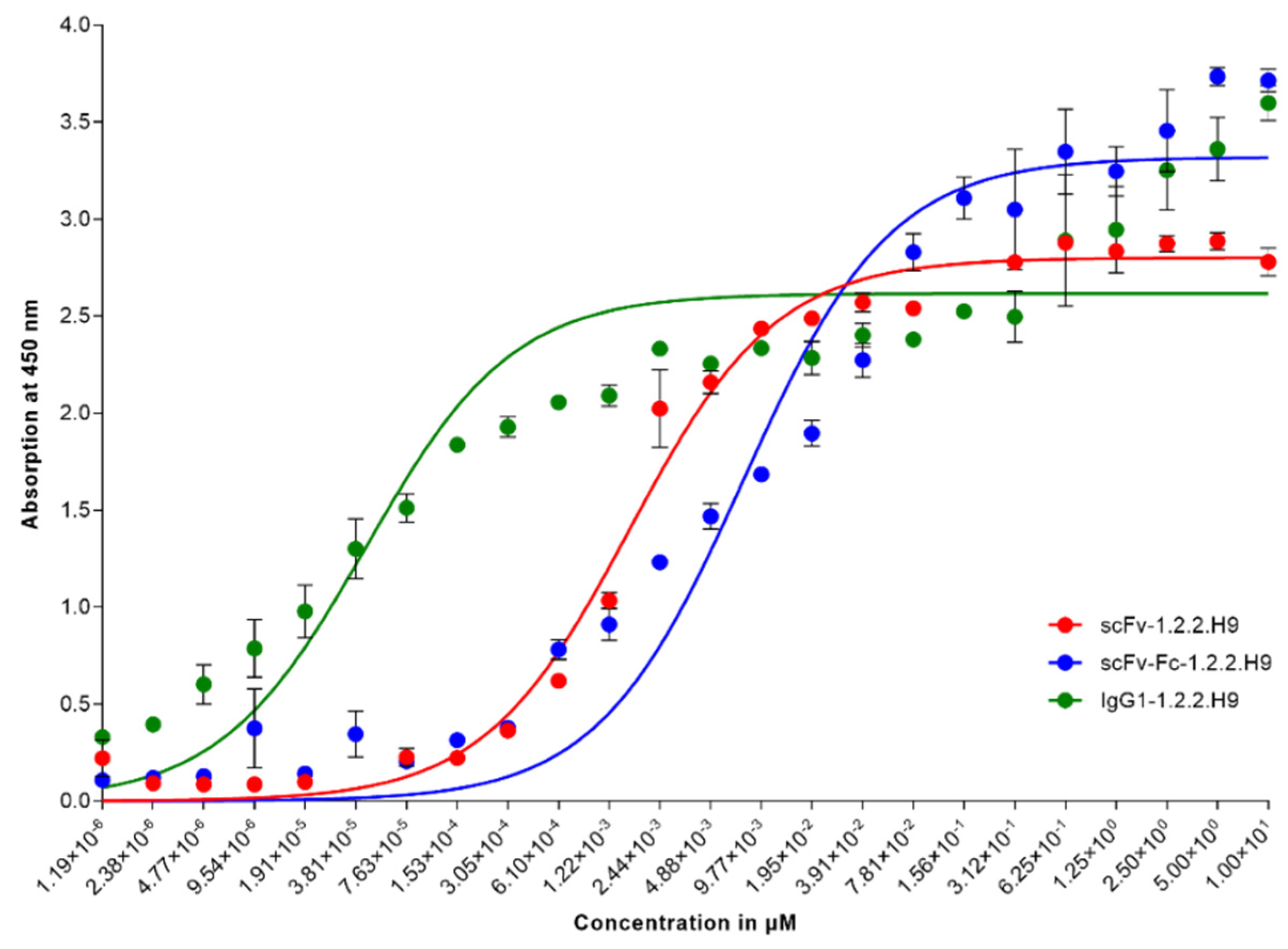
Vaccines Free Full Text Characterization Of An In Vivo Neutralizing Anti Vaccinia Virus D8 Single Chain Fragment Variable Scfv From A Human Anti Vaccinia Virus Specific Recombinant Library Html

How To Prepare Standardization 0 1 N Sodium Hydroxide Naoh In 2021 Sodium Sodium Hydroxide Preparation
Carbonate Bi Carbonate Coating Buffer Ph 9 6 Medicago

0 05 M Edta Solution Preparation Standardization In 2021 Solutions Preparation Stirrers
Molecules Free Full Text Gc Ms Analysis Of Biological Nitrate And Nitrite Using Pentafluorobenzyl Bromide In Aqueous Acetone A Dual Role Of Carbonate Bicarbonate As An Enhancer And Inhibitor Of Derivatization Html

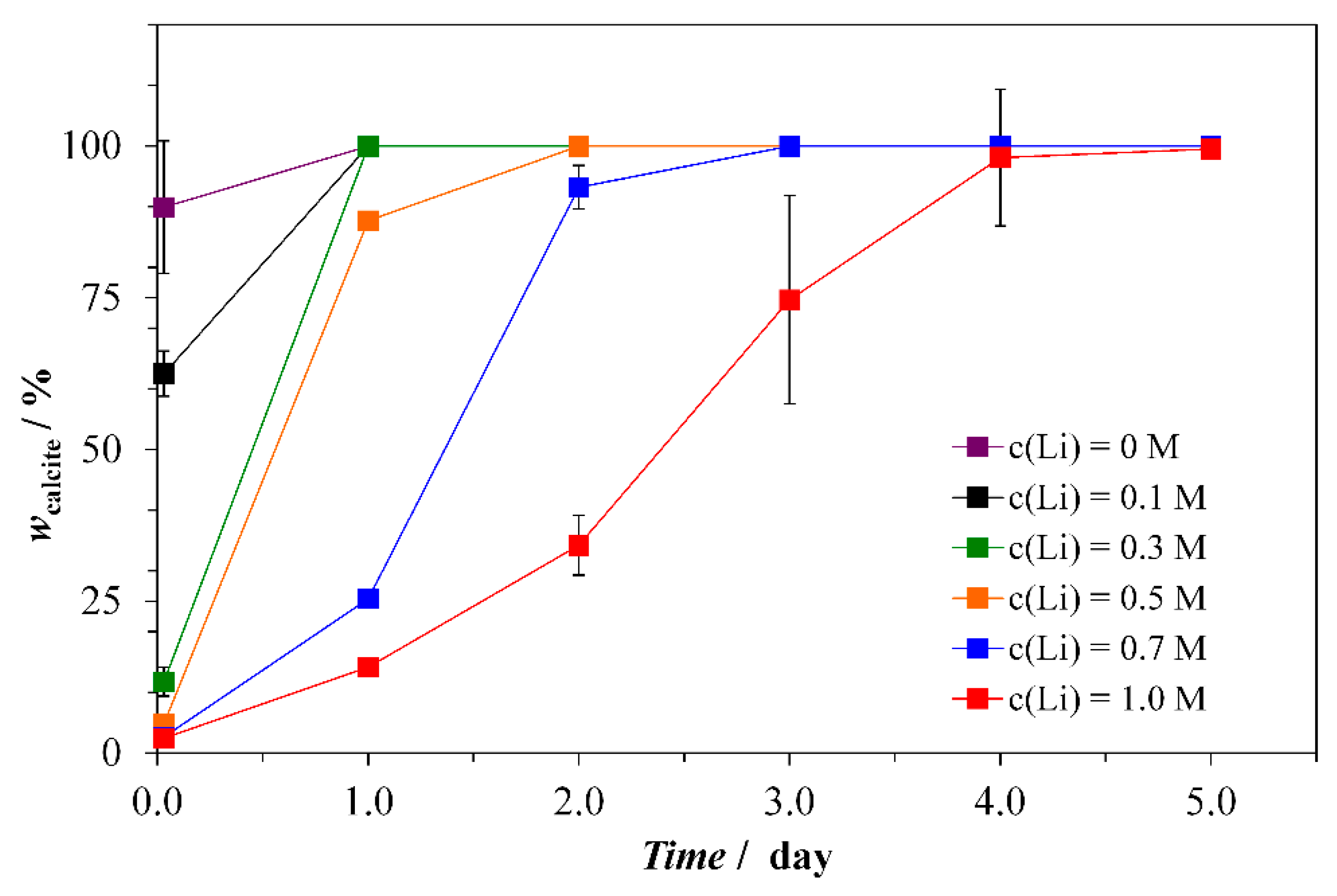
Crystals Free Full Text Synthesis And Adsorbing Properties Of Tabular 001 Calcite Crystals Html

0 1m Sodium Bicarbonate Sodium Carbonate Buffer Ph 9 6 0 2

How To Prepare Standardization 0 1 N Iodine Solution In 2021 Iodine Solutions Amber Glass Bottles



Posting Komentar untuk "0.05 M Carbonate-bicarbonate"