Carbonate Buffer System In The Body
The bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. This buffer system is essential because exercise produces carbon dioxide and lactic acid in muscles.
The bicarbonate buffer system is what the body uses.

Carbonate buffer system in the body. The role of the bicarbonate buffer system in regulating blood pH This is the currently selected item. When atmospheric carbon dioxide is dissolved in seawater carbonic acid H 2 CO 3 is formed. Other buffers perform a more minor role than the carbonic-acid-bicarbonate buffer in regulating the pH of the blood.
While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. Control system CO2 and H are potentially toxic products of aerobic and anaerobic metabolism most CO2 is lost through the lungs but some is converted to bicarbonate Thus contributing important extracellular buffering capacity Bicarbonate system is the most important buffer in the body because has high capacity. The bodys chemical buffer system consists of three individual buffers.
A buffer system has the property of resisting pH changes despite additions of acid or base. A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3. The optimal pH level of the blood is 74 which is maintained by three different types of buffer systems working in the body 2The addition of an acid or a base to a substance changes its pH level.
How does a carbonate buffer system work. The bodys chemical buffer system consists of three individual buffers. A buffer system exists to help neutralize the blood if excess hydrogen or hydroxide ions are produced.
In the human stomach and duodenum the bicarbonate buffer system serves to both neutralize gastric acid and stabilize the intracellular pH of epithelial cells via the secretion of bicarbonate ion into the gastric mucosa. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO 2 and hydrogen ions respectively from the body. In the body a bicarbonate buffer system is used to regulate the pH of blood.
This question now b. A buffer system in the human body is an interaction between a weak acid-base conjugate pair that keeps the. In humans and other animals the carbonate buffering system helps maintain a constant pH in the bloodstream.
Changes in hydrogen carbonate ion concentration however require hours through the relatively slow elimination through the kidneys. The bodys chemical buffer system consists of three individual buffers. Carbonic acid is diprotic which means in has two H ions to donate to solution.
The Carbonic AcidBicarbonate Buffer. The bicarbonate buffering system is an crucial buffer system in the acid-base homeostasis of all living things. An important buffer system in the human body is the bicarbonate buffering system that keeps human blood in the right pH range.
The bodys chemical buffer system consists of three individual buffers. The pH of blood depends on the ratio of carbon dioxide to bicarbonate. It can be shown as.
Haemoglobin is an important blood buffer particularly for buffering CO2. The carbonate and phosphate buffer systemsthese are not in the pH range necessary to buffer blood. The phosphate buffer consists of phosphoric acid H 3 PO 4 in equilibrium with dihydrogen phosphate ion H 2 PO 4- and H.
The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteinsWhile the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. What is the chemical equation for the carbonic acid buffer system. The phosphate buffer system is NOT an important blood buffer as its concentration is too low.
While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. The main role of the bicarbonate system is to regulate and control the pH of blood and counteract any force that will alter the pH. The carbonic acid-hydrogen carbonate ion buffer works throughout the body to maintain the pH of blood plasma close to 74.
The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. The three major buffer systems of our body are carbonic acid bicarbonate buffer system phosphate buffer system and protein buffer system. True False Increasing alveolar ventilation increases extracellular fluid H concentration and decreases pH.
Carbon dioxide plays a vital role in the chemistry of sea water. Other pH-Buffer Systems in the Blood. The three major buffer systems of our body are carbonic acid bicarbonate buffer system phosphate buffer system and protein buffer system.
Carbonic acid H2CO3 a compound. Both components must be present for the system to act as a buffer to resist changes in pH. The pK for the phosphate buffer is 68 which allows this buffer to function within its optimal.
The salt is the conjugate of the weak acid or of the weak base. The bicarbonate buffer system is an effective buffer system despite having a low pKa because the body also controls pCO2. There are other uses but thats general knowledge of chemical solutions not a physiological phenomenon or system.
Other mechanisms that assist in this function include the hemoglobin molecule in your red blood cells which also helps to buffer blood pH. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. When the first proton is donated HCO3- otherwise known.
Using optical traps to manipulate single DNA strands. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. A buffer is a solution of a weak acid or a base and its salt.
The bodys chemical buffer system consists of three individual buffers out of which the carbonic acid. Also what are the chemical buffers in the body. Buffer Systems in the BodyThe buffer systems functioning in blood plasma include plasma proteins phosphate and bicarbonate and carbonic acid buffersThe kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range.
The pH or the amount of hydrogen ions H in a solution level of the blood is important in ensuring the proper functionality of biological systems 2. Carbonic acid concentration is controlled by respiration that is through the lungs. The CarbonateBicarbonate Buffer System.

Solaray Super Bio Vitamin C Time Release 360 Vegcaps Vitamins Vitamin C Digestion Process
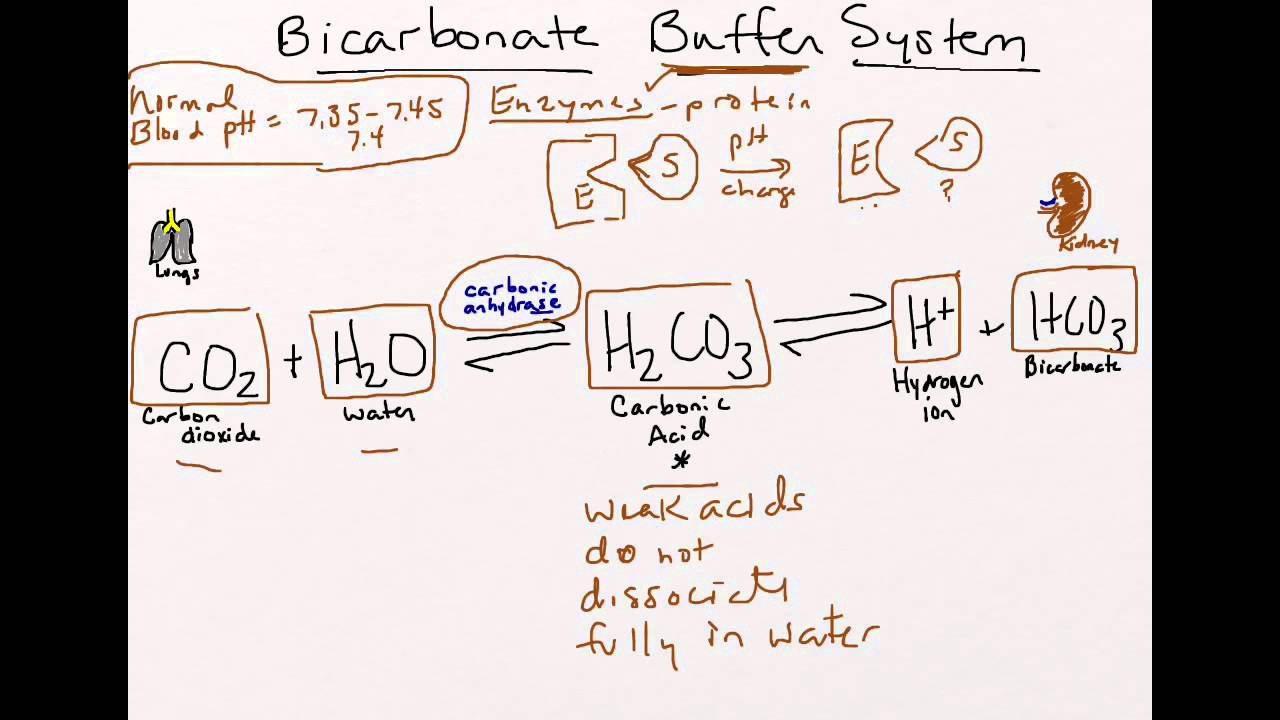
Bicarbonate Buffer System System Study Tips Pharmacy School

What Is The Best Aquaponics Fish To Plant Ratio Get To Know The Answer In Our Blog Post Aquaponicsfish Aquaponics Fish Backyard Aquaponics Aquaponics System

Natural Factors 100 Natural Fruit Chew Vitamin C Blueberry Raspberry And Boysenberry 500 Mg 180 Chewable Wafers Fruit Chews Chewable Vitamins Chewable Vitamin C

Country Life Buffered Vitamin C 1000 Mg 250 Tablets Vitamins Vitamin C Country Life

Country Life Maxi Hair Plus 5000 Mcg 120 Vegetarian Capsules Gluten Free Hair Products Biotin Vitamins

Vitafusion Extra Strength D3 Bone Immune Support Natural Strawberry Flavor 75 Mcg 120 Gummies In 2020 Gummies Flavors Culinary Chef

Water Problem Solutions Aquaponics Water Treatment Water Well

Pin On Chemistry Lab Techniques






Posting Komentar untuk "Carbonate Buffer System In The Body"