Hydrogen Carbonate Ion Lewis Structure
Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule with resonance forms where appropriate. Complete octets on outside atoms.

Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 Molecular Geometry Writing Chemistry
Consider the bicarbonate ion also called the hydrogen carbonate ion.

Hydrogen carbonate ion lewis structure. Hydrogencarbonate is the carbon oxoanion resulting from the removal of a proton from carbonic acid. It forms two single bonds with two OH ions while a double bond with the remaining oxygen bond. The main structure is derived from resonance structures are termed as resonance hybrid.
B three single bonds around the central carbon atom. Cycloalkanes are rings of hydrocarbon structures that possess three alkane compounds thus being saturated. A two double bonds around the central carbon atom.
This is probably from the electrons of sodium calcium or whatever salt resulted in a cation that the donated electrons to the carbonate anion. H always goes outside. A step-by-step explanation of how to draw the HCO3- Lewis Structure Hydrogen Carbonate or Bicarbonate IonYou should put the HCO3-Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule. Steps for Writing Lewis Structures. For the H CO 3-Lewis structure Carbonic Acid make sure you put the Hydrogen atom on the outside of an oxygen atoms.
Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Carbonate lewis structure and bicarbonate lewis structure In carbonate ion there is two oxygen atoms which has -1 charge on each of them. Hco3- Lewis Structure.
It is a conjugate base of a carbonic acid. Carbonate ion lewis structure. Put two electrons between atoms to form a chemical bond.
Each hydrogen atom group 1 has one valence electron carbon group 14 has 4 valence electrons and oxygen group 16 has 6 valence electrons for a total of 21 4 6 12 valence electrons. The remaining 14 electrons are distributed around the oxygen centres ie. It is represented by several resonance structures which contribute to the main structure of compound.
85 436 ratings FREE Expert Solution. After drawing the correct Lewis dot structures you would see. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule with resonance forms where appropriate.
Hydrogen carbonate ion. Formal Charges And Resonance Chemistry Atoms First. NOCl CF 2 Cl 2 HCN.
85 436 ratings Problem Details. Of one central carbon atom and three oxygen atoms. Lewis Structure Of Hydrogen Carbonate Ion HCO3 Lewis Structure.
It has a role as a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite a mouse metabolite and a cofactor. With H CO 3- Carbon C is the least electronegative and goes in the center of the structure. D two equivalent resonance forms.
C four single bonds around the central carbon atom. The bicarbonate ion hydrogencarbonate ion is an anion with the empirical formula HCO 3 and a molecular mass of 6101 daltons. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
Compare the electron dot structures of the carbonate CO 3 2- and borate BO 3 3- ions. Because carbon is the least electronegative element we place it in the central position. The Carbonate CO_32 Ion.
It is a conjugate acid of a carbonate. Draw the Lewis structure of bicarbonate HCO 3- showing all possible resonance structures if there are any. One of these oxygen atom take a proton H ion and form a -OH group.
Find the total valence electrons for the molecule. After counting the valence electrons we have a total of 9 5 from nitrogen 41 from each hydrogen 9. CO32- Lewis Structure - How to Draw the Lewis Structure for CO3 2- Carbonate Ion - YouTube.
H 2 S NCl 3 OH-Put the least electronegative atom in the center. If an H ion attaches to CO 3 2-to form the bicarbonate ion HCO 3- does it attach to an O atom or to the C atom. You should put the H CO 3-Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Steps for Writing Lewis Structures. A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure Hydrogen Carbonate or Bicarbonate IonFor the HCO3- structure use the periodic tabl. Determine the formal charge of each atom.
Determine the total number of valence electrons in the molecule or ion. How to Draw the Lewis Structure for Formal Charge Problems 3 Carbonate CO3 YouTube Common Polyatomic Ions StudyBlue Formal Charges and Resonance Chemistry for Majors. How many resonance structures do each ion have.
And 1 each from the negative charge and hydrogen. Write the Lewis structure for the ammonium ion NH 4. It consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with a hydrogen atom attached to one of the oxygens.
Hydrogen atoms are always placed on the outside of the molecule so nitrogen should be the central atom. Introduce an hydrogen ion carbonate becomes bicarbonate and the Lewis structure is OC-O-OH. There are 24 valence electrons involved in the bonding.
Toothpastes containing sodium hydrogen carbonate sodium bicarbonate and hydrogen peroxide are widely used. CH 4 NH 3 I 2. Determine the formal charge of.
Explore the various shapes sizes and names of alkanes as they bond to form. A molecule or ion which contains delocalized electrons and cannot be represented by single Lewis structure. What are the formal charges of each atom in these ions.
E three equivalent resonance forms. Are these ions isoelectronic.

Hco3 Lewis Structure How To Draw The Electron Dot Structure For The Hydrogen Carbonate Ion

Ch3br Lewis Structure Bromomethane In 2021 How To Find Out Molecules Lewis
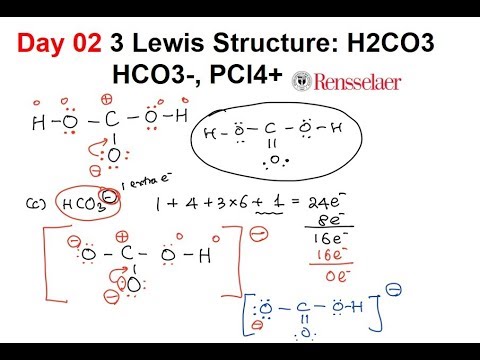
Day02 3 Lewis Structure H2co3 Hco3 Pcl4 Youtube

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion In 2021 Molecular Geometry Molecular Geometry

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com
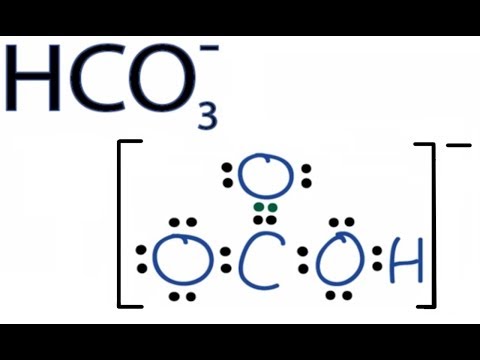
Hco3 Lewis Structure How To Discuss
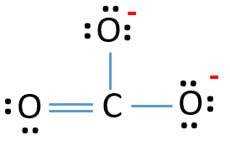
Lewis Structure For Co32 Carbonate Ion

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Boron

Is Pcl3 Polar Or Non Polar Phosphorus Trichloride In 2021 Phosphorus Chemical Formula Molecules

Ch3och3 Lewis Dot Structure Diethyl Ether In 2021 Functional Group How To Find Out Molecules

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
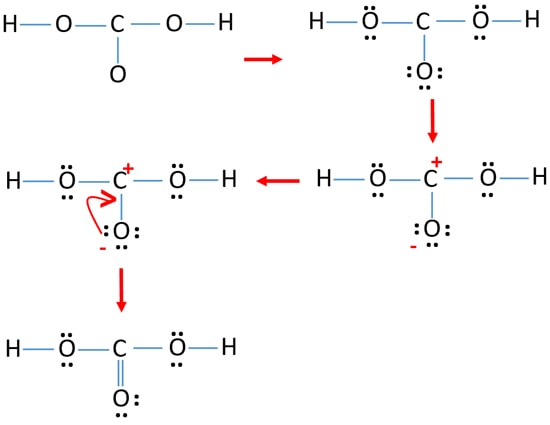
H2co3 Carbonic Acid Lewis Structure
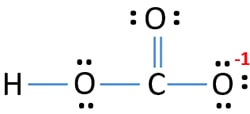
Bicarbonate Hco3 Ion Lewis Structure
Posting Komentar untuk "Hydrogen Carbonate Ion Lewis Structure"