Carbonate Ion Shape And Bond Angle
In this instance one must include all resonance forms of the lewis structure in resonance structures for carbonate co32 Lewis structures are diagrams of molecules that represent electron pairs by dots crosses or lines. The two C-O single bonds and the CO double bond.

A Level Gce Shapes Of Molecules Appendix Shapes Of Oxyanions Ks5 As A2 Revision Notes
What is the hybridization of bromine in bro2.

Carbonate ion shape and bond angle. In certain molecules axial and equatorial positions are unique. H 2 O 2 bonds. The central carbon atom in the carbonate CO32- ion is sp2 hybridised.
Use bond angles in PCls to identity and describe. To be the center atom ability of having higher valance is important. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions but it gives.
The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Hence Sulfur Hexafluoride has sp3d2 hybridization.
From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds called the bond angles. Net Dipole Moment and 2- Charge. I cant think of any case where carbonate ion acts as a Lewis acid.
Since there are only three bonds attached to it and no lone pairs. CO32- Molecular GeometryShape and Bond Angles Carbonate Ion CO32- ion comprises one Carbon atom and three Oxygen atoms along with two additional electrons. Carbonate ion CO 3 2-is trigonal planar in shape with a O-C-O bond angle of 120 o because of three groups of bonding electrons and no lone pairs of electrons.
Analyze the bonds in the carbonate ion. Bond angles also contribute to the shape of a molecule. Center atom of CO 3 2-ion.
Therefore its polar bonds are distributed evenly. Carbonate ion has one carbon atom three oxygen atoms and -2 charge. There are no lone pairs of electrons which would repel with the surrounding atoms and provide a different bond angle and shape.
The bond angle of F-S-F is 90 degrees. CO32- is carbonate ion. The given chemical species is ammonium ionNH4 N H 4 and it is a polar compound.
In this video we find out the molecular geometry of this ion by looking at its Lewis Structure its shape in 3D and the steric number. CO32- is a type of structure that has a trigonal-planar shape and a bond angle of 120 degrees. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells.
The shape and geometry of the ion are tetrahedral so that the dipole moments of the N-H polar bonds are equal and opposite to each other and thus cancel each other. Total electron pairs are determined by dividing the number total valence electrons by two. Iii There are three possible Lewis structures that can be drawn for the carbonate ion which lead to a resonance structure.
The hybridization of bromine must be sp3. The shape of the carbonate ion is given by. This results in equivalent bond angles of 120 making the structure symmetrical in nature.
Bond angles are the angles between adjacent lines representing bonds. Explain with reference to the electrons why all carbon-oxygen bonds have the same length. The lewis structure of the carbonate ion has two single bonds to negative oxygen atom and carbonate uses.
The net dipole moment for the molecule is zero so it is non-polar. H 3 O 3 bonds. The bond angle can help differentiate between linear trigonal planar tetraheral trigonal-bipyramidal and octahedral.
The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize. What contributes to this shape. The shape is deduced below using dot and cross diagrams and VSEPR theory and illustrated below.
The ion is planar with a bond angle of 120 0. Specifically compare the standard bond lengths for C- and CO in Tables A and 2 with the WebMO-computed bond length Table 3. What do you infer about the CO bonds in the carbonate ion.
It can accept a proton H to form bicarbonate ion HCO3- making carbonate ion a Bronsted-Lowry base. From this we can describe the molecular geometry. The CO32- ion therefore has a trigonal-planar shape just like BF3 with a 120 degree bond angle.
The only thing that contributes to this shape are the three bonds which the central atom is attached to. As Sulphur shares its valence electrons with 6 Fluorine atoms we can see that all six electrons of the Sulphur atom are shared to form bonds. But these electrons are concentrated in three places.
The VSEPR theory therefore predicts that CO 2 will be a linear molecule just like BeF 2 with a bond angle of 180 o. Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP. As shown above the CO 3 2-ion has symmetrical nature.
Thus there is a presence of one double bond in the carbonate ion. Since the proton binds by accepting an electron pair from carbonate ion to form a bond carbonate ion is also a Lewis base. What is the bond angle in the carbonate ion CO32-.
For CO 3 2-ion Total pairs of electrons are 12. It has a Trigonal Planar geometry. Iv Deduce the hybridization of the carbon atom in the carbonate ion.
14 Carbonate Lewis Structure. These six orbitals are in the six directions of the octahedron shape.
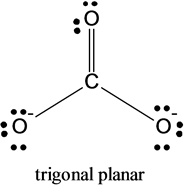
Predict The Molecular Shape Of Methane The Carbonate Ion Carbon Dioxide And The Sulfite Ion Home Work Help Learn Cbse Forum

Valence Bond Structure Of Carbonate Ion Co3 2 Download Scientific Diagram
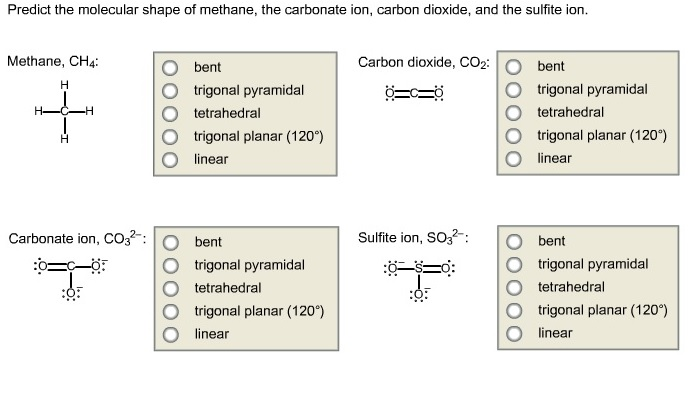
Solved Predict The Molecular Shape Of Methane The Carbonate Chegg Com

Calcium Carbonate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

A Level Gce Shapes Of Molecules Appendix Shapes Of Oxyanions Ks5 As A2 Revision Notes

Hybridization As A Way Of Explaining Vsepr Theory Ppt Download

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Is Co32 Polar Or Nonpolar Carbonate Ion Polarity Geometry Of Molecules
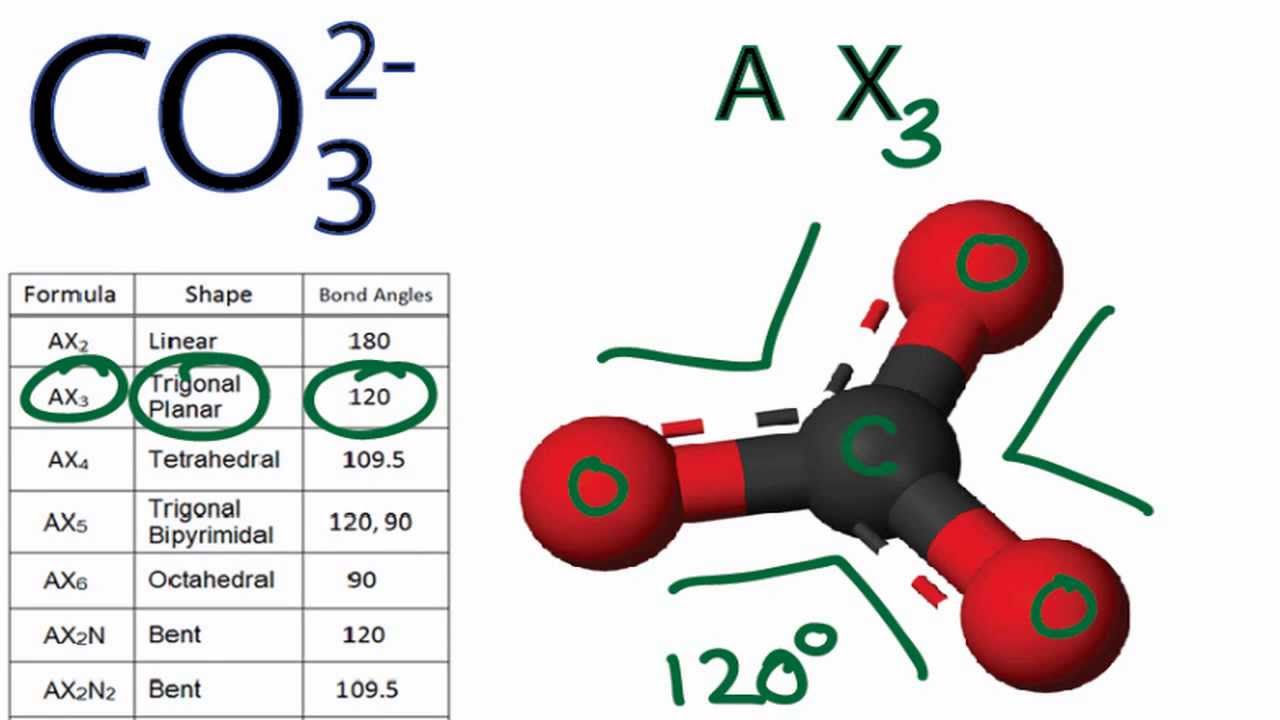
Co3 2 Molecular Geometry Shape And Bond Angles Youtube

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion Youtube
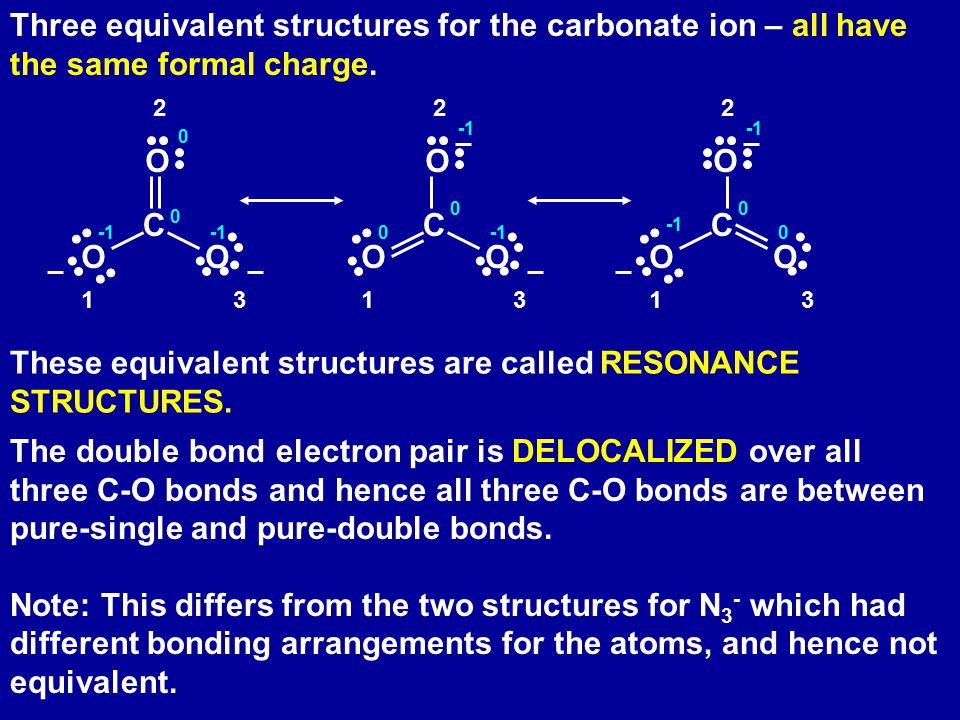
Example Co3 Carbonate Ion Ppt Download

A Level Gce Shapes Of Molecules Appendix Shapes Of Oxyanions Ks5 As A2 Revision Notes
Posting Komentar untuk "Carbonate Ion Shape And Bond Angle"