Carbonate Ion Bond Length
Here the bonds are not fixed and show resonance therefore all C-O bonds are equal in length. There are three σ bonds and π bond around carbon atom in the Lewis structure of CO 3 2-ion.
It is this additional pi bonding which makes each of the carbon-oxygen bonds have a bond order of 133 and a bond length and bond strength between those of.
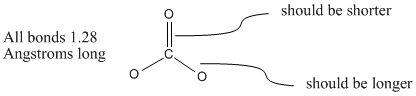
Carbonate ion bond length. Carbonates are readily decomposed by acids. But it has been found that all the three bonds are of the same length and this observation is attributed to the resonance effect. In acetylene HCCH the carbon-carbon bond order is also 3 and the CH bond order is 1.
Use bond angles in PCls to identity and describe. Carbon-oxygen bonds are of equal length in carbonate ion. Carbonate ion all 3 C-O bonds have identical length - - Carbonate ion has two C-O single bonds and 1 CO double bond which suggests CO bond is shorter than two C-O bonds.
All the CO bonds in carbonate ion CO 3 2 are equal in length. Carbonate ion C O 3 2 shows resonance and thus all the three bonds are of identical bond length. Why is there difference in bond enthalpy of OH bond in ethanol C 2 H 5 OH and water.
Metal carbonate compounds are common in the world. Bond length in decreasing order. Single bond double bond triple bond.
In ion carbon is bonded to 3 oxygen atoms. The bond length depends on the size of the atom and the number of bonds multiplicity between the combining atoms. As an CaCO 3 can be given.
Asked by Archit 12102018 Last Modified 13102018. Compare Calculated Bonds for O-C. In certain molecules axial and equatorial positions are unique.
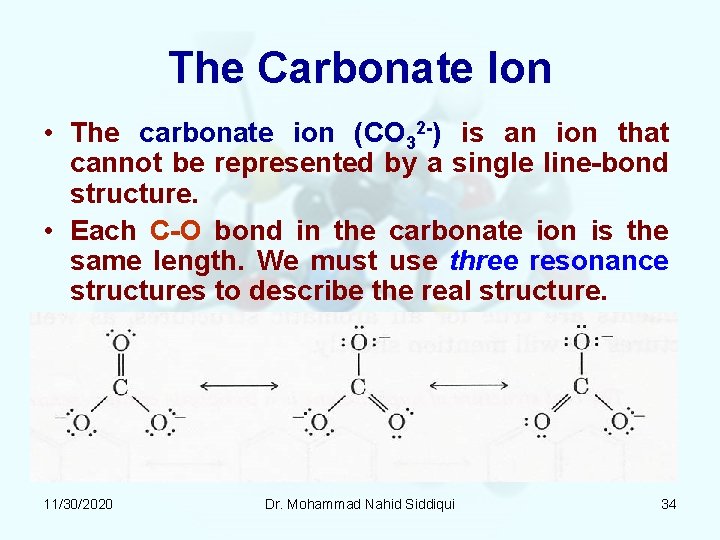
TPSSh6-31G2dfp Histogram of Bond lengths in Å vs number of species Values greater than 230 are in the 230 bin. Carbonate Ion is a polyatomic ion with formula of CO3 2-. What do you infer about the CO bonds in the carbonate ion.
For example in diatomic nitrogen NN the bond order is 3. Steps of drawing lewis structure of CO 3 2-. - But experimentally all 3 bonds have identical length.
You cannot draw a Lewis structure that would suggest all three bonds are the same length. Match the species in Column I with the type of hybrid orbitals in Column II. It is a conjugate base of a hydrogencarbonate.
Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. Average Bond Order Formula. Carbonate is a carbon oxoanion.
Carbonate ion CO 3 2-Carbonate ion has a -2 charge. Specifically compare the standard bond lengths for C- and CO in Tables A and 2 with the WebMO-computed bond length Table 3. CBSEClass 11ScienceChemistryChemical Bonding and Molecular Structure CBSEClass 11ScienceChemistry.
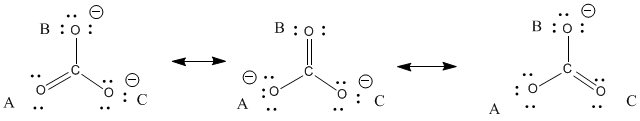
The carbonate ion according to its Lewis di agram has two types of carbon -oxygen bonds one double bond and two single bon ds suggesting that one carbon -oxygen bond in the carbonate ion is shorter and stronger than each of the other two. - This is because it can be represented by 3 Lewis structures. All three carbon-oxygen bond distances are about 128 Angstroms long.
The bond length of C-O bond is 142 pm CO is 116 pm whereas the bond length of carbonate ion is 131 pm. What is meant by the term average bond enthalpy. For example carbon-carbon single bond length 154 Å is longer than the carbon-nitrogen single bond length 143 Å.
Analyze the bonds in the carbonate ion. Hope this helps Mark as brainliest. Why are C-O bond lengths in a carbonate ion identical.
Bond length decreases with the multiplicity of bond between two atoms. The bond order is 2 not 4 for ceCO2 and the bond order for the carbonate ion is somewhere between 1 and 2 due to resonance. Greater the size of the atom greater will be the bond length.
Thus the carbonate ion has the longest bond length followed by carbon dioxide and finally carbon monoxide. One of the 3 oxygens is enaged in the double bondand the other two oxygens connected to craboon by a single sigma bond each hold the negative charge of one electron excess each. Was this answer helpful.
Seeing the structure one would imagine that in carbonate ion two bonds will be the same in length the two single bonds and will be longer as compared to the third bond which is a double bond. It is bonded to 2 oxygen atoms by double bond. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond.
Instead you can use resonance structures to understand this fact. Bond order and bond length indicate the type and. Carbonate ion is a symmetric trigonal planar molecule.
Average Bond Order is given by rmAveragermbondrmorder fracrmNumberrmofrmbondsrmNumberrmofrmresonancermstructures. All the C O bonds in carbonate ion CO 2-3 are equal in length. Bond Order and Lengths.
Column I Column II. Experimenta lly however the three carbon -oxygen bonds in the carbonate ion have the same bond length. CO 3 2-Lewis structure.
Each structure has alternating double and single bonds but experimentation shows that each carboncarbon bond in benzene is identical with bond lengths 1399 pm intermediate between those typically found for a CC single bond 154 pm and a CC double bond 134 pm. The carbon in Carbonate ion is in an sp2 hybridisation and there are 3 sigma bonds and one pi bond around it.

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram
Why Are The Bond Lengths In Carbonate Ion Equal Quora
Solved 6 Use The Resonance Structures Below For The Chegg Com

Lecture 22 C Slg Chm 151 Resonance Octet Violators Formal Charges Molecular Shapes Topics Ppt Download
Chapter 2 Orbitals And Their Role In Covalent
All The C O Bonds In Co3 2 Are Equal In Length Why Quora

All The C O Bonds In Carbonate Ion Co3 2 Are Equal In Length Explain

In Carbonate Ion All The Three C O Bonds Have Identical Bond Length Explain Brainly In

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Chapter 6 Resonance And Electron Delocalization Chapter 6 Topics For Test U Sections 6 1 Through 6 13 U I Will Emphasize Drawing Resonance Structures Ppt Download

Lecture 22 C Slg Chm 151 Resonance Octet Violators Formal Charges Molecular Shapes Topics Ppt Download

Posting Komentar untuk "Carbonate Ion Bond Length"