Carbonate Buffering
Carbon dioxide plays a vital role in the chemistry of sea water. The pH of blood depends on the ratio of carbon dioxide to bicarbonate.

Coccolithophore Carbon Chemistry Carbon Cycle Diatom Ocean Acidification
Carbon dioxide from the tissues diffuses rapidly into red blood cells where it is hydrated with water to form carbonic acid.

Carbonate buffering. Carbonate buffers are a combination of a weak acid and its conjugate salt relying upon series of reverse reactions to buffer changes in pH. Limestone is not a good source of alkalinity for recirculation aquaculture because the dissolution of limestone by carbonic acid. Carbonate buffer concentrate for use with 2725 Methanol Membranes and 2786 Ethanol Membranes.
We made the choice to try a Carbonate buffer because it has a reported pH buffering range of 51-71 and the seperation needs to be performed at or around pH57. Ridgwell. Carbonate buffering can enhance or inhibit the stream CO 2 evasion by regulating stream CO 2 pool in an indirectly manner especially in high-alkalinity streams Duvert et al 2019.
Use caution in handling sodium azide as it is. The major extracellular buffer in the blood and the interstitial fluid of vertebrates is the bicarbonate buffer system. 497-19-8 Sodium Carbonate Anhydrous Reagent Grade 08499A 1 Kg - MW.
One that is important in surface waters is the carbonic acidbicarbonate buffer. Potassium Chloride KCl 11-18. The carbonate buffering term was obtained by first calculating CH for all river and groundwater locations based on our measurements of pCO 2 and DIC.
The best part of adding sodium bicarbonate is. Carbonate Buffered solution 01M Sodium Bicarbonate-Sodium Carbonate Buffer pH 90 UPR16490 250 ml - Applications. Buffer solutions EUROPEAN PHARMACOPOEIA 60 Sodium standard solution 1000 ppm Na.
Buffer pKa and pH Range Values For preparation of. Immediately before use dilute. Reconstitute to make 1 liter.
Well in any buffer system the boost in. When atmospheric carbon dioxide is dissolved in seawater carbonic acid H 2 CO 3 is formed. Lowering pH brings many advantages but none of which are easily described but basically the biggest example is that at lower pH a developer becomes less sensitive to CO2.
The bicarbonate and carbonate ions are responsible for the buffering capacity of seawater ie. 16 g Na2CO3 15 mM final 29 g NaHCO3 35 mM final 02 g NaN3 31 mM final. This reaction is accelerated by carbonic anhydrase an enzyme present in high concentrations in red blood cells.
1579 Carbonate Buffer Concentrate. Buffers pKa range. Carbonate-Bicarbonate Buffer pH 92 to 106 preparation guide and recipe.
Stets et al 2017. When pH is low the concentration of hydrogen ions is too high so one exhales CO 2. That is it can undergo two de-protonation reactions to form bicarbonate HCO 3 - and carbonate CO 3 2-.
Perchloric Acid HClO. Other mechanisms that assist in this function include the hemoglobin molecule in your red blood cells which also helps to buffer blood pH. Click to see full answer.
In natural systems there are many buffers. The carbonate buffering system coupled with control of CO 2 exchange allows the aquaculturist to do this relatively easily. For instance carbonic acid H 2CO 3 and sodium bicarbonate NaHCO 3 or even sodium bicarbonate and calcium carbonate.
Buffer edit It works as a buffer in the blood as follows. Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings. Sodium standard solution 200 ppm Na.
In humans and other animals the carbonate buffering system helps maintain a constant pH in the bloodstream. Dissolve a quantity of anhydrous sodium carbonate R equivalent to 2305 g of Na2CO3 in a mixture of 25 ml of water Rand 25 ml of nitric acid R and dilute to 10000 ml with water R. Important organocarbonates include dimethyl carbonate the cyclic compounds ethylene carbonate and propylene carbonate and the phosgene replacement triphosgene.
I am trying to use a 25mM Sodium Carbonate buffer in a seperation I am developing. What happens when you titrate this combination with the strong acid of your choice. Carbonic acid is diprotic which means in has two H ions to donate to solution.
We took on average that the best position for buffering at 20 deg C for the CarbonateBicarbonate was at 101. Buffers in the pH. You will see that in use in the RA and C-41 developers most notably.
Recipe can be automatically scaled by entering desired final volume. Coating protein oon microplates. Carbonate buffers Theory A classic buffer is a combination of a weak acid and its conjugate salt.
A Brief Summary of Carbonate Buffer System Chemistry Atmospheric CO 2 dissolves in seawater and is hydrated to form carbonic acid H 2 CO 3. Hydrochloric Acid - HCl 0-2. Oxalic Acid C.
Nitric Acid - HNO. Heterogeneous buffering in the ocean is dominated by carbonate compensation Berner 2004. To make 1 liter of carbonate buffer add the following.
Sodium Carbonate Anhydrous ACS grade 141321 1 Kg 141322 25kg 141321 MW. Description of carbonate buffering how it effects concentration of carbon dioxide in the atmosphere and changes the pH of the oceansThank you for watching. The CarbonateBicarbonate Buffer System.
Hey all - I have a question about carbonate buffers. Carbonate Buffers Recall that buffers are mixtures of weak acids and their conjugate bases that resist changes in pH. The carbonate ion can react with calcium ions Ca which are in excess in.
6 where K 0 corresponds to the temperature-dependent Henrys law constant for CO 2 molm 3 atm 1 and CH and DIC are expressed in molm 3 and p CO 2 in atm. The inorganic carbon equilibrium CO2HCO3CO3 is the MAIN buffering system in fresh water so if you add more bicarbonate then you increase the buffering capacity of the system. When the first proton is donated HCO3- otherwise known.
Carbonic acid is divalent. Seawater can resist drastic pH changes even after the addition of weak bases and acids.

44lb Hydra Ph Buffer Keep Balance General Hardness Carbonate Hardness By Hydra 109 95 Use In Ponds With Vin Vinyl Liners Water Gardens Pond Ponds Backyard

Aquaponics Ph Explained Finding The Perfect Balance Aquaponics Balance Explained Finding Perfect Backyard Aquaponics Aquaponics Diy Aquaponics
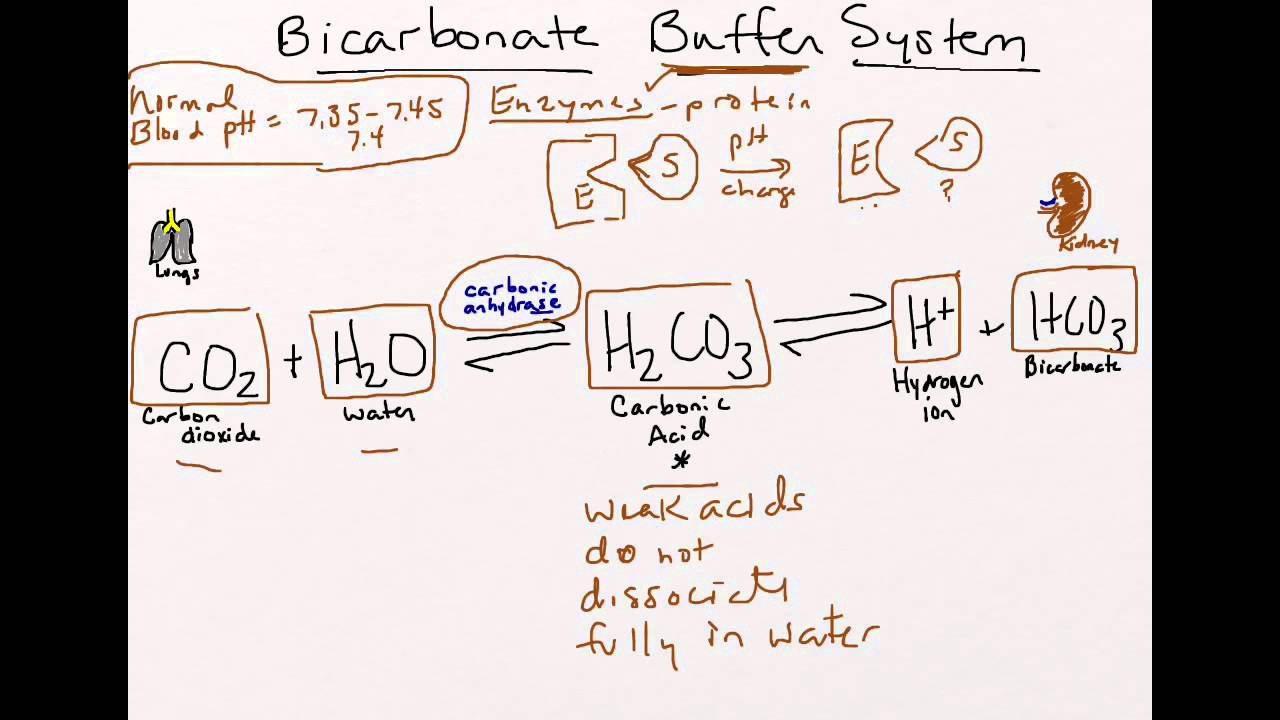
Bicarbonate Buffer System System Study Tips Pharmacy School

Multimedia Surface Tension Water And Detergent Surface Tension Middle School Chemistry Multimedia

Aquaponics Ph Explained How To Maintain The Perfect Balance Aquaponics Solid Shampoo Bar Well Water System

Specific Plant Ph Infographic Hydroponicsinfographic Hydroponicstips Hydroponicgardening Hydroponicsorgan Aquaponics System Aquaponic Gardening Hydroponics

Carbonate Bicarbonate Buffer Ph 9 2 To 10 6 Preparation Guide And Recipe Recipe Can Be Automatically Scaled By Entering D Preparation Recipes Chemistry Labs

Hydroponic Gardening Plant Nutrients Plants Hydroponic Gardening

General Hydroponics Ph Control Kit For A Balanced Nutrient Solution In 2021 Hydroponics Hydroponics Diy Gallon





Posting Komentar untuk "Carbonate Buffering"