Carbonate Ion Lewis Structure Resonance
This gives rise to three resonance forms of the carbonate ion. Lets consider the Lewis structure of the carbonate ion CO32.

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube
Steps of drawing lewis structure of CO 3 2-Following steps are required to draw the CO 3 2-lewis structure and they are explained in detail in this.
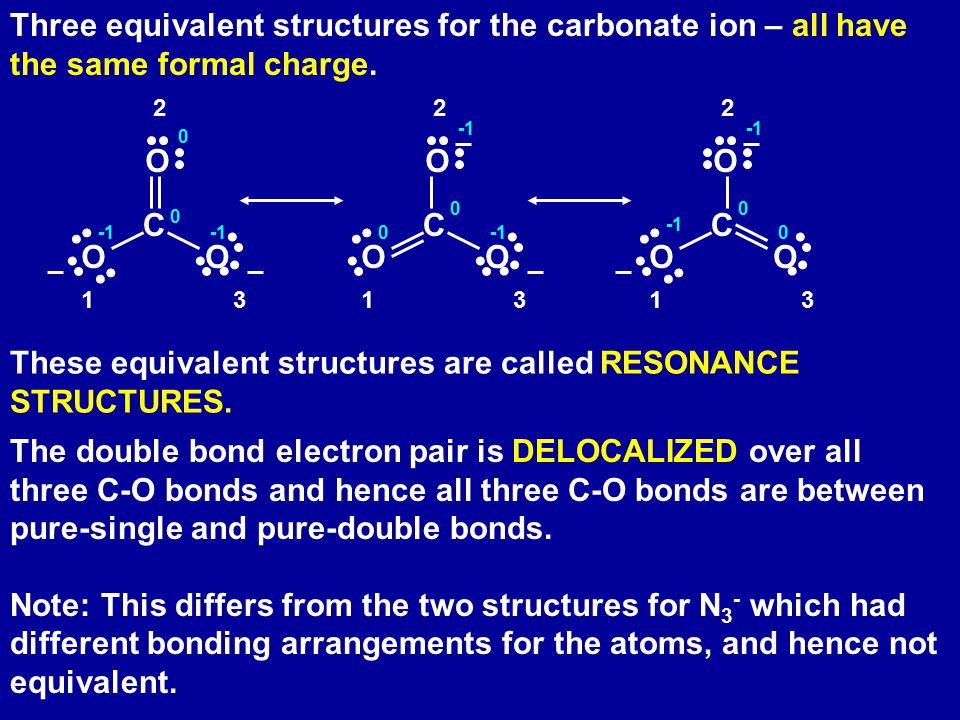
Carbonate ion lewis structure resonance. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. The lewis structure of the carbonate ion has two single bonds to negative oxygen atom and carbonate uses. There are three different possible resonance structures from carbonate.
14 Carbonate Lewis Structure. Has one carbonoxygen double bond and two carbonoxygen single bonds. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
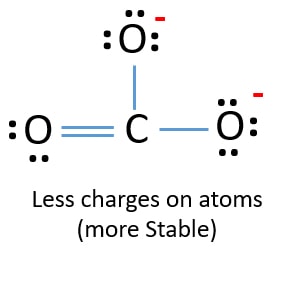
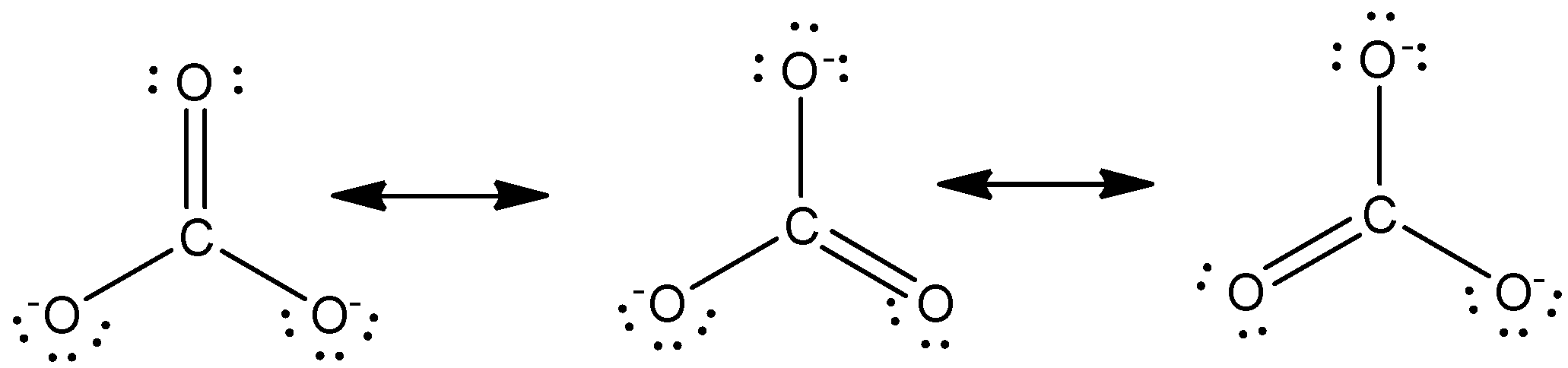
Because we can write three identical resonance structures we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. In this question we have to describe the resonance structure of carbonate ions. Unlike O3 though the actual structure of CO32 is an average of three resonance structures.
Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. 91 470 ratings FREE Expert Solution. However carbonate ion is an exception to the rule.
It is evident from the experimental results that all carbon-oxygen bonds in carbonate ion are equivalent. It is also made to describe the manner of bonding in a particular ion or molecule by merging many structures called resonance structures. Each of the singly bonded.
In this regard how many Lewis structures does co32. Is CO3 2 an acid or base. Instead you can use resonance structures to understand this fact.
Draw the Lewis structure of the carbonate ion CO32- showing all possible resonance structures if there are any. The correct Lewis structure for this ion has one carbonoxygen double bond and two carbonoxygen single bonds. These three resonance structures.
You cannot draw a Lewis structure that would suggest all three bonds are the same length. But which of the three. Oxygen atoms bears a formal charge of 1 and all other atoms are neutral.
In this instance one must include all resonance forms of the lewis structure in resonance structures for carbonate co32 Lewis structures are diagrams of molecules that represent electron pairs by dots crosses or lines. In fact carbonate ion is a symmetric trigonal planar molecule. The correct Lewis structure for this ion.
Carbonate ion a moderately strong base undergoes considerable hydrolysis in aqueous solution. Because carbon is the least electronegative element we place it in the central position. Resonance structures are shown by polyatomic molecules or ions in which sets of lewis structure shows delocalization of electrons.
Lewis structure - resonance carbonate Polyatomic ion whose formula is HCO3- trigonal planar. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. By signing up youll get.
Unlike O 3 though the actual structure of CO 32 is an average of three resonance structures. Such structures are called resonance structures and this phenomenon is called resonance. All oxygen atoms however are equivalent and the double bond could form from any one of the three atoms.
Return to Lewis Structures. There are three σ bonds and π bond around carbon atom in the Lewis structure of CO 3 2-ion. Carbon has 4 valence electrons each oxygen has 6 valence electrons and there are 2 more for the 2 charge.
How many resonance forms are possible. A molecule or ion through such delocalized electron is stood for by numerous contributing frameworks also dubbed resonance structures or canonical forms. One may also ask is co3 2 a resonance structure.
Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Resonance is a method of relenten delocalized electron within details molecules or polyatomic ions where the bonding can not be to express by a single Lewis formula. CO 3 2-Lewis structure.
The actual structure of the molecule is said to be a resonance hybrid an average of these 3 resonance forms. Because of that this is the best Lewis structure for CO3 2-. The Carbonate C O 3 2 Ion.
Unlike O 3 though the actual structure of CO 32 is an average of three resonance structures. Metal carbonate compounds are common in the world. As an CaCO 3 can be given.
Each carbon oxygen bond can be thought of as 1333 bonds. A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu. Carbonate ion has a -2 charge.
All three carbon-oxygen bond distances are about 128 Angstroms long. The Carbonate CO23 Ion Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. How many resonance structures does co32.
The average of a double bond and 2 single bonds. Draw the Lewis structure for the carbonate ion CO32-. Carbonate ion resonance structure of carbonate ion CO32 CO32 molecule ion drawn of SO 3 2-ion this ion has one carbonoxygen double bond lone.
The Carbonate CO23 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. CO3 2 NO3 and O3.

Resonance Structures Of Co3 2 The Carbonate Ion Youtube
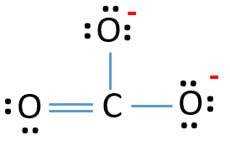
Lewis Structure For Co32 Carbonate Ion

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube

Example Co3 Carbonate Ion Ppt Download
Lewis Structure For Co32 Carbonate Ion
Write The Resonance Structure Of Carbonate Ions Class 12 Chemistry Cbse
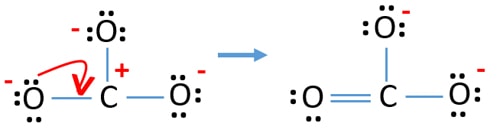
Lewis Structure For Co32 Carbonate Ion
What Is The Lewis Structure Of Co3 2 Quora

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Consider The Resonance Structures For The Carbonate Ion Image Src Charge5986180229036662068 Jpg Alt Charge Caption Study Com
Posting Komentar untuk "Carbonate Ion Lewis Structure Resonance"