Carbonate Ion Formal Charge
CO32- Lewis Structure - How to Draw the Lewis Structure for CO3 2- Carbonate Ion - YouTube. The formal charge on carbon atom in carbonate ion is.
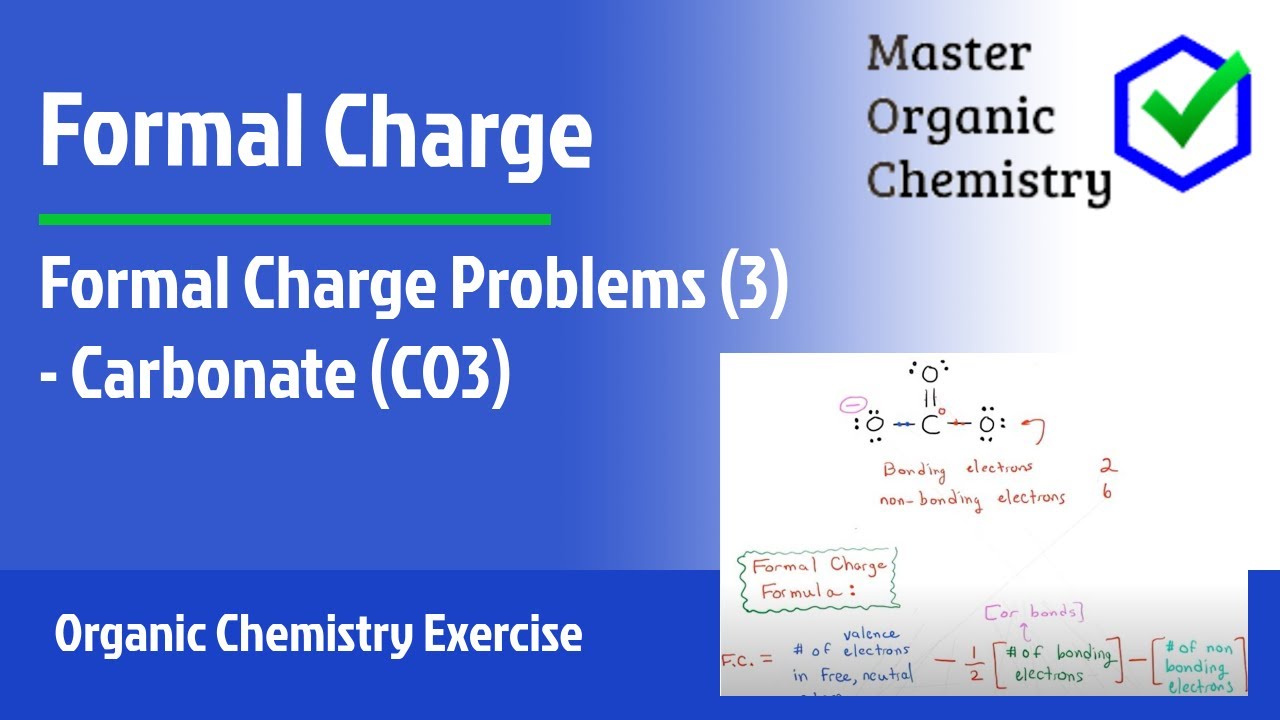
Formal Charge Problems 3 Carbonate Co3 Youtube
The Carbonate CO_32 Ion.

Carbonate ion formal charge. The formal charge of carbon in carbonate ion is 0. And total no of bonding electrons8. Formal charge valence - non bonded - 12 total number.
Each oxygen has a formal charge of 23 and the carbon has a formal charge of zero. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Using the structure of carbonate ion calculate the formal charge of a central carbon atom.
Formal charge is calculated using F C V N B 2. The formal charge of carbon atom in carbonate ion is-. It is giving overall results of 426 electrons.
The formal charges on the atoms in both these ions may be calculated as follows. The formal charge on the central xenon atom in. We will have to know that formal charge of an atom is not equal to real charge of an ion or a molecule.
It has a molecular mass of 6001 gmol and carries a total formal charge of 2. Formal charge is only a theoretical charge over an individual atom of an ion a molecule. The oxygen in carbonate ion has 4x14 electrons and assigned to it and from one from each four bonds.
It consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D3h molecular symmetry. In order to calculate the formal charges for CO32- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e. Formal charge on carbon 4 0 8 2 0 The formal charge of any atom in a molecule can be calculated by the following equation.
Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. C O 3 2 already has a charge of 2-. We can convert each lone pair to a bonding electron pair which gives each atom an octet of electrons and a formal charge of 0 by making three CC double bonds.
Open Answer in App. In order to calculate the formal charges for HCO3 - well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e. Formula for Carbonate.
It has D3h molecular symmetry and is made up of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement. The conjugate base of H. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
Carbonate ion CO 3 2-Carbonate ion has a -2 charge. I n carbonateone oxygen is bonded to carbon with double bond and two other oxygens are bonded to carbon with single bondSo Structural formula for carbonate is given below. Now let us calculate the formal charge of the oxygen atom which is.
The structure of carbonate ion is the simplest oxocarbon anion. I Formal charge on atoma in carbonate ion. After finishing the lewis structure of CO 3 2- there should be a -2 charge and it should be stabile structure.
It can be said that the carbonate ion has a charge of 2 and each of the single bonded oxygen atoms holds a charge of 1. First we calculate the number of bonds needed. Three carbon atoms now have an octet configuration and a formal charge of 1 while three carbon atoms have only 6 electrons and a formal charge of 1.
NCI Thesaurus NCIt Carbonate is a carbon oxoanion. It has a total formal charge of 2 and a molecular mass of 6001 gmol. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams.
Salts or ions of the theoretical carbonic acid containing the radical CO2 3. F C V N 2 B Was this answer helpful. Thus the formal charge of oxygen in carbonate ions is zero.
Metal carbonate compounds are common in. So the carbonate ion carbon atom is connected with two oxygen atoms with the help of single bonds and with one oxygen atom by double bond as shown above. You will learn about these facts in this tutorial.
The carbonate ion is the simplest oxocarbon anion. In carbonate ionthe oxidation number of carbon is 4 and the oxidation number of O is -2The net charge of carbonate is -2. It is a conjugate base of a hydrogencarbonate.
It has been taken with formal charge of zero neutral and includes at top oxygen that has two lone pairs as well as bonding pairs.
Calculating Co32 Formal Charges Calculating Formal Charges For The Carbonate Ion Youtube

Formal Charge On Carbon Atom In Carbonate Ion Is Chemistry Electrochemistry 14010889 Meritnation Com
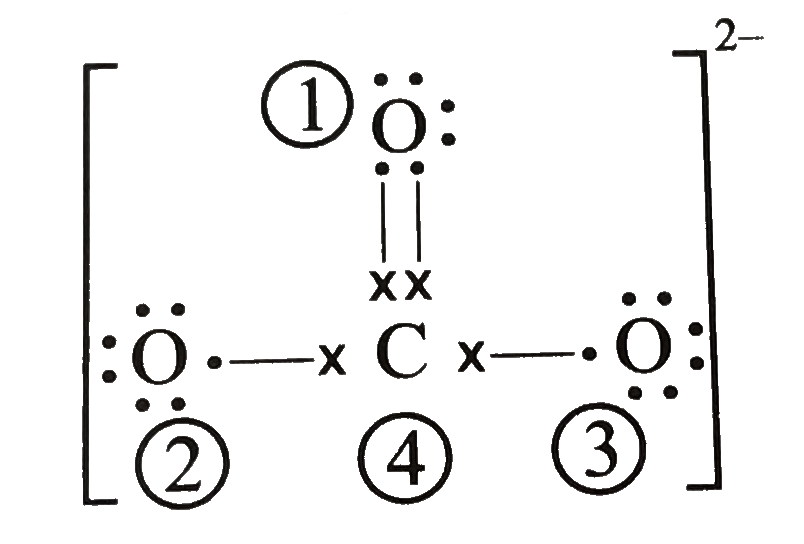
Calculate The Formal Charge On Atoms In Carbonate Co 3 2
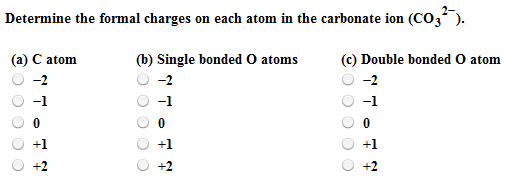
Solved Determine The Formal Charges On Each Atom In The Chegg Com
What Is The Lewis Structure Of Co3 2 Quora
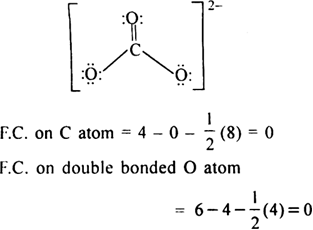
Calculate The Formalcharge On Atoms In Carbonate Ion From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
Download 35 Possible Resonance Structures For Co32

What Is The Formal Charge On Atom In Carbonate And Nitric Acid

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
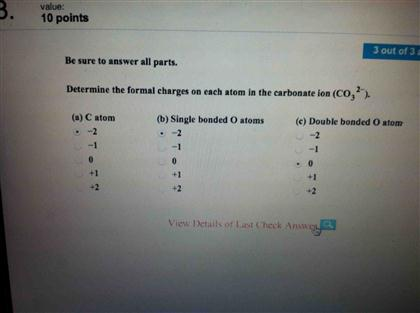
Solved Determine The Formal Charges On Each Atom In The Chegg Com
How To Calculate The Formal Charges For Co3 2 Carbonate Ion Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Formal Charge Problems 3 Carbonate Co3 Youtube

Formal Charge Problems 3 Carbonate Co3 Youtube
Posting Komentar untuk "Carbonate Ion Formal Charge"