Carbonate Base Equation
Carbonate group COO 2. Calcium carbonate occurs naturally as chalk limestone and marble.

Chemistry Metal Carbonate And Hygrodencarbonates Acids Bases And Salts Part 2 English Youtube
The hydrogen ions H from the acid react with the carbonate ions CO 3 2- to form water and carbon dioxide gas.

Carbonate base equation. Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. Thus for the carbonate system were worried about here if you want to keep the same pH but halve or double the KH you would expect to halve or double CO 2 to keep the same ratio and the same equilibrium pH. The photo shows the reaction with marble chips.
Can you remember what it was. Metal carbonate sulfuric acid metal sulfate carbon dioxide water. The sodium bicarbonate is decomposed by heat producing sodium carbonate.
HCl aq NH 3 aq NH 4 Cl aq You should notice that in the first two examples the base contained OH ions and therefore the products were a salt and water. Carbonates are moderately strong bases. It could react as a acid with a base.
So by definition of a Lewis base it attracts protons in aqueous solutions. Carbon dioxide is then bubbled through to precipitate the bicarbonate NaHCO3. Carbonate is a weak bae and baes do not react with other bases.
CaO CO 2 s. Potassium carbonate sulfuric acid potassium sulfate carbon dioxide water. General equation for the reaction of an acid with a metal carbonate.
It consists of an atom surrounded by three oxygen atoms. Carbonic acid dissociates into bicarbonate and carbonate according to the following equations. It is also found in many forms such as marble limestone etc.
And if it takes a hydrogen from water H2O there will be a hydroxide ion OH- left over. Metalcarbonate acid to salt water carbon. The traditional Solvay process is utilized in most parts of the world.
NaCl table salt and KBr. A salt is also produced. The general equation for the reaction between an acid and a base is as follows.
Carbonic acid H 2 CO 3 is the acid and water is the base. It can be made as the product of potassium hydroxide s absorbent reaction with carbon dioxide. Reactions involving calcium carbonate.
H 3 O K a HA A-. Hydrogen carbonate is a weak base and csnnot react as a base but it is also a weak acid. It presents a large capacity to absorb moisture.
Sodium carbonate is a diazonium salt of carbonic acid with chemical formula Na2CO3. Carbonate is a polyatomic ionIt has -2 chargeIn carbonatewe get one carbon and. The easiest way to calculate the pH based on selected ion concentrations is the Henderson-Hasselbalch equation.
However about 70 of the world production capacity of sodium carbonate is manufactured by the Solvay ammonia soda process whereby ammonia is added to a solution of sodium chloride. The conjugate base for H 2 CO 3 is HCO 3-bicarbonate ion. Aqueous solutions are basic because the carbonate anion can accept a hydrogen ion from water.
The equilibrium on the left is an acid-base reaction that is written in the reverse format from Equation 3. In its pure form it is white powder and odourless. When you put sodium carbonate Na2CO3 in water it produces 2 sodium ions and one carbonate ion CO3 with a charge of 2-.
Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solution. It is found in the earths crust. The only place CO3 can get hydrogen ions from is water.
But wait there was a third product in our reaction. Today I will show you the formula for carbonate in this blog post. The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid.
If we replace base with metal carbonate we get. Substitute the name of each reactant and each product into the general word equation. Acid base salt water.
Carbonate Alkalinity CA CA 2CO 3-2 HCO 3- Typically HCO 3-and CO 3-2 are present at 1000x conc of other proton acceptors Hence. The chemical formula for calcite. The aqueous solution of sodium carbonate Na 2 CO 3 is basic in nature due to having more hydroxide ions produced from the hydrolysis of carbonate ions CO32- H2O HCO3 OH.
This inorganic compound is water-soluble and when dissolved in water it forms carbonic acid and sodium hydroxide. Ammonium chloride is made up of NH 4 cations from the base NH 3 and Cl anions from the acid HCl. Carbonate acid salt carbon dioxide water.
Sodium Carbonate Formula. By knowing the K a of the acid the amount of acid and the amount of conjugate base the pH of the buffer system can be calculated. It may be an anion and includes a trigonal planar molecular structure.
For example a solution of sodium hydrogen carbonate reacts with a sodium hydroxide solution to form sodium carbonate. HCl H2O H3O Cl-H2O H2O H3O OH-H2CO3 H2O H3O HCO 3-In the first reaction HCl transfers a proton to H2O. OH-aq HCO3-aq H2Ol CO32-aq.
In addition it may be a moderately strong base. CA nearly equals TA Calculations Any two of the four CO 2 properties ΣCO 2 P CO2 pH and carbonate alkalinity can be used to determine the CO 2 system Traditionally pH and alkalinity. Carbonate rock weathering of is major global factor in natural water acid-base chemistry.
It is also known as Soda crystals soda ash washing soda. Acid1 Base1 Proton Proton Base2 Acid2----- Acid1 Base2 Acid2 Base1 For example. Acid metal carbonate salt water.
Note that water H2O in the second reaction can be both an acid proton donor and a base proton acceptor. Acid carbonate salt CO 2 water. 2 3 -3 o - 64 H CO 2 3 H HCO K 10 H CO 3 where H 2 CO 3 H 2 CO 3 CO 2 aq H 2 CO 3 Note that Ko H2CO3 210 -4 or pK 369 if corrected for CO 2aq.
CO32 H2O HCO3 OH Carbonates react with acids forming salts of the metal gaseous carbon dioxide and water. Na 2 CO 3 is a basic salt having a pH value close to 11 made from the neutralization of a strong base NaOH with a weak acid H 2 CO 3. CaCO 3 sthe principle mineral in limestonecan be reformulated as a sum of oxides.
Reacts with a base such as. The above equation for K a can be rearranged to solve for the hydronium ion concentration. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms where the use of chalk a form of CaCO3 is found.

Which Formula Can Be Used To Calculate The Exact Hydronium Concentration Present In Sodium Hydrogen Carbonate Solution Chemistry Stack Exchange
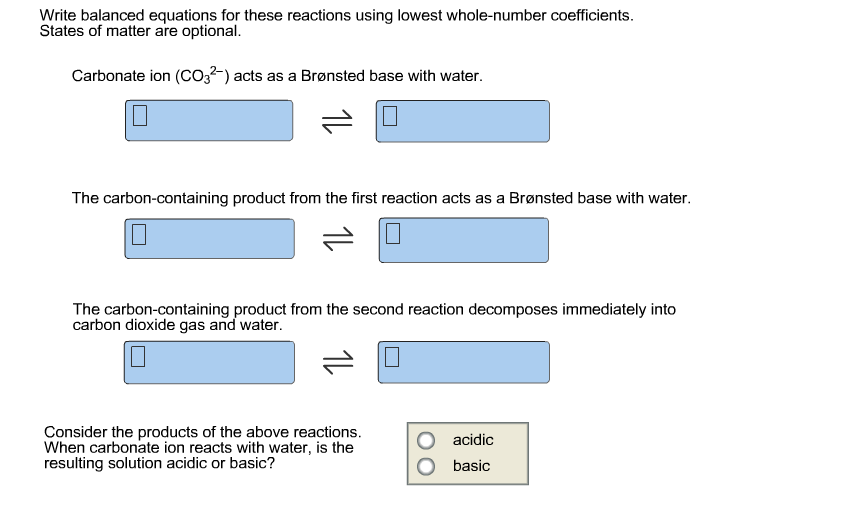
Solved Write Balanced Equations For These Reactions Using Chegg Com

Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube

11 7 Reactions Of Acids And Bases Ppt Download

Equation For Sodium Carbonate Dissolving In Water Na2co3 H2o Youtube
Chem 101 Acids And Bases Introduction
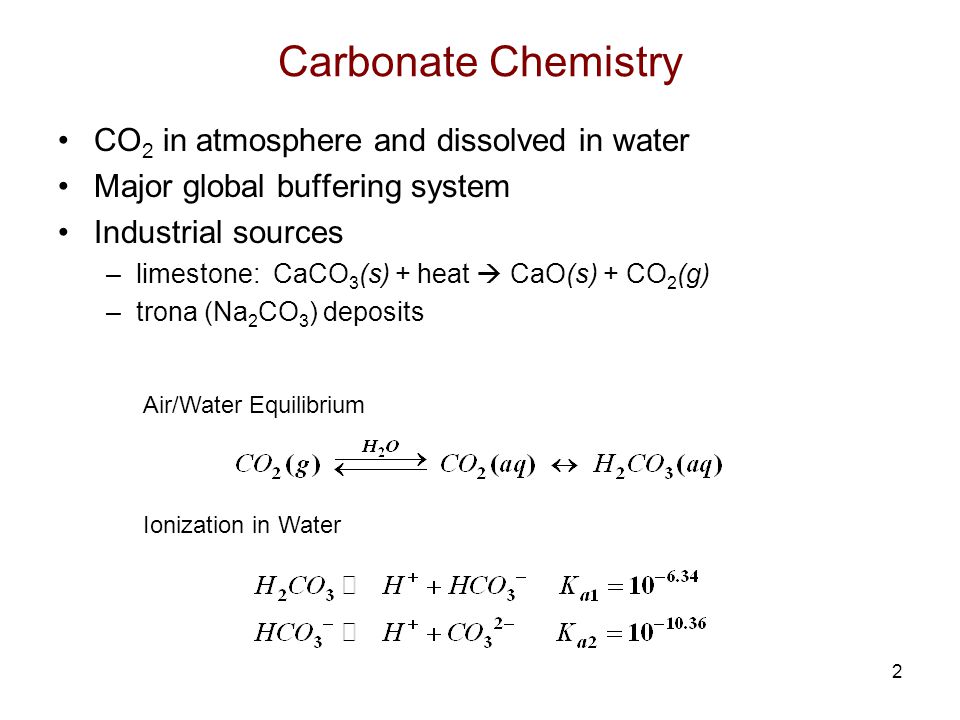
Titration Of Sodium Carbonate Ppt Video Online Download

Acids Bases And Salt Preparations Gcse The Science Hive
Volkscience Chemistry Made Easy Short Tutorial Acid Base Reactions Ionic Equations Units 2 4 I Ve Recently Had A Few Students Asking Me About Acid Base Reactions So Here Is A Not So
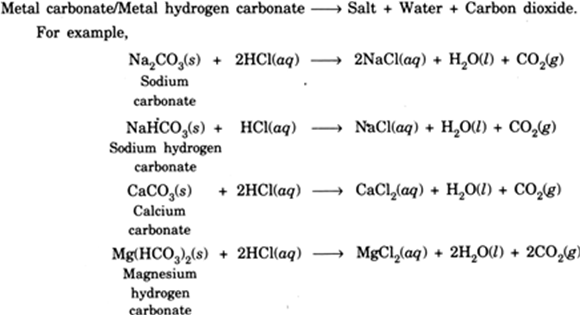
Name The Gas Evolved When A Metal Carbonate Or Metal Hydrogen Carbonate Reacts With Acids Explain The Chemical Reaction From Science Acids Bases And Salts Class 10 Haryana Board English Medium

Is Na2co3 Acidic Basic Or Neutral Dissolved In Water Youtube

Identify The Acid And Base Which Form Sodium Hydrogen Carbonate Write Chemical Youtube
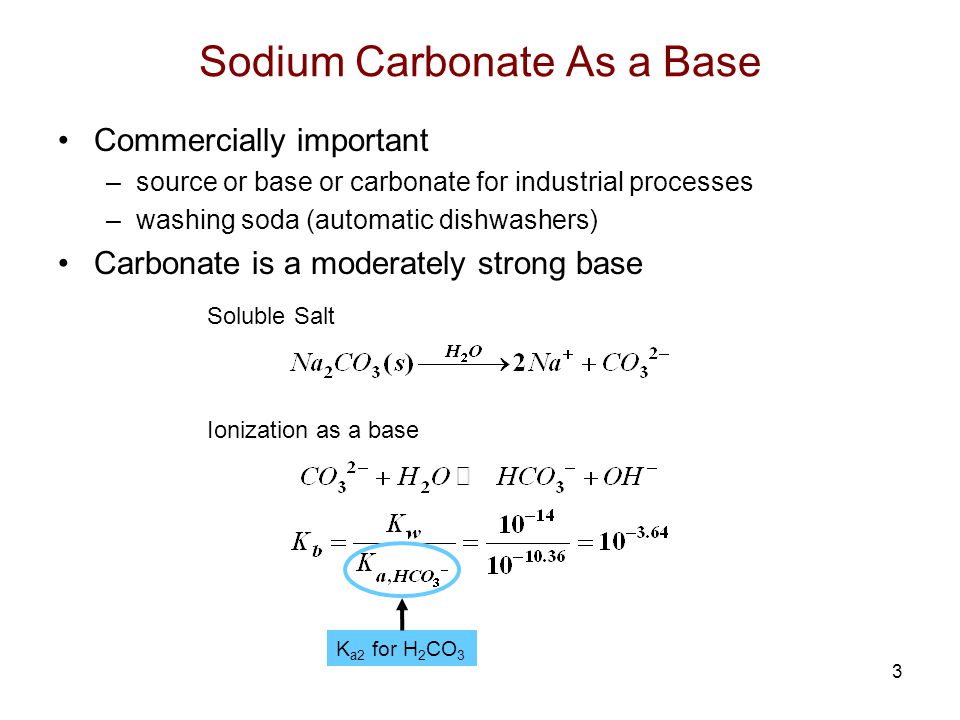
Titration Of Sodium Carbonate Ppt Video Online Download
Posting Komentar untuk "Carbonate Base Equation"