A Carbonate Not Decomposed By Heat
I suppose that some alkali metal carbonates do not decompose even in bunsen burner flame. Most of the metal carbonates decompose on heating.
Why Is Li2co3 Decomposed At A Lower Temperature Whereas Na2co3 At Higher Temperature
The total mass of the black residue formed was 796 g.
A carbonate not decomposed by heat. This is because sodium carbonate salt on heating with acids react to. All carbonates decompose on heating. Calcium carbonate decomposes at much lower temperatures.
Other metal carbonates decompose in the same way. The bonds of sodium bicarbonate is so strong that too much energy is required to break them down. Click here to get an answer to your question 1.
Na 2 CO 3 s Na 2. This experiment can be carried out conveniently in. A nitrate which produces oxygen as the only gas.
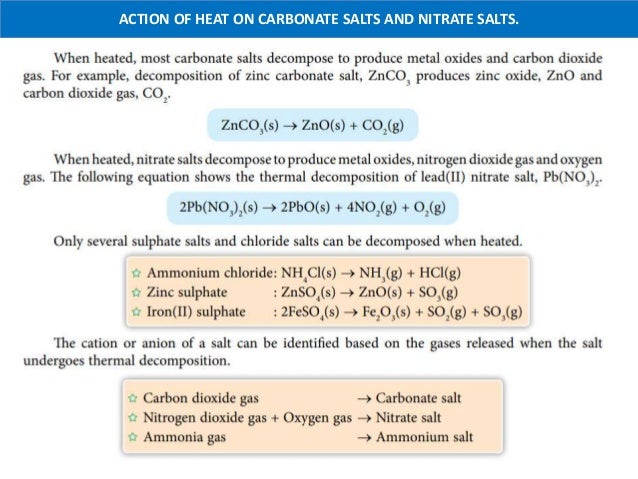
Nitrate salts also undergo decomposition on heating. Anhydrous sodium carbonate is stable to heat and does not decompose even when it is heated to redness. Similarly why does potassium carbonate not decompose when heated.
Some carbonates are more reactive than others. Sodium carbonate and potassium carbonate is stable and it doesnt decompose by heat. Here are the equations that represent the thermal decomposition of calcium carbonate.
On the other hand we see that as Sodium and Potassium belongs to alkali metals or group I metals so the carbonates formed with the alkali group metals will not decompose on heating as they are thermally stable except Li_2CO_3 which is thermally unstable. Sodium Carbonate is more readily decomposed than Calcium Carbonate. You may need some of the information in the table.
This is because lithium carbonate is covalent. Lithium ion being very small in size polarizes large carbonate ion leading to the formation of more stable lithium oxide. Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide.
Sodium Carbonate and Potassium Carbonate. It is not realistically possible to measure the enthalpy change of this reaction directly but using the fact that both calcium carbonate and calcium oxide react directly with hydrochloric acid a Hess law cycle can be. Action of Heat on Nitrate Salts.
Table below shows the effect of heating on metal carbonate. Copper carbonate is easily decomposed by heating. Simply so which carbonates do not decompose on heating.
Here are some examples. Calcium carbonate is decomposed on heat-ing in the following reactionCaCO CaO CO1. If you want to explore decomposition temperatures of carbonates you need very strong heating.
Carbon dioxide gas CO2 16. The aim of this experiment is to compare the reactivity of some different metal carbonates. Sodium carbonate sodium oxide carbon dioxide.
Why sodium carbonate does not decompose on normal heating. Sodium Nitrate and Potassium Nitrate. When metal carbonates are heated they break down to form the metal oxide and carbon dioxide gas.
What mass of calcium carbonate is this. Group 1 metal carbonates are not decomposed by heat. A carbonate which does not decomposes on heating.
During an investigation a scientist heated 1236 g of copper carbonate till it decomposed to form a black residue. The maximum temperature that a bunsen burner could provide is 11001200oC 1100 1200 o C. We can start from the basic conception of heat stability of ionic compounds it mainly depends on the lattice enthalpyenergy required to break 1 mole of a compound in positive and negative ions.
Therefore lithium carbonate decomposes at a low temperature while a stable sodium carbonate decomposes at a high temperature. The calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater. A carbonate not decomposed by heat is sodium bicarbonate.
Sodium carbonate Na2CO3 17. Since sodium carbonate is very stable it decomposes at high temperature. Most metal nitrates decompose to produce a metal oxide nitrogen dioxide and oxygen.
2 on a question. However lithium carbonate is not so stable to heat. How many moles of C.
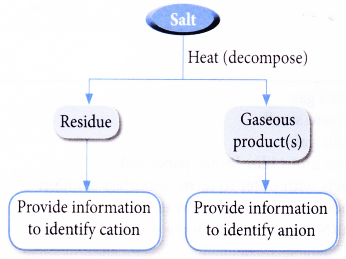
What gas is released when carbonate salts are decomposed by heat. 05 moles of calcium carbonate are decomposed by heating. Examples are sodiumcarbonate calcium carbonate potassium carbonate uranylcarbonate magnesium carbonate.
For high lattice enthalpy in a compound it becomes hard to break the bonds between negative and positive ions. EXPERIMENT 67 INVESTIGATE THE ACTION OF HEAT ON CARBONATE SALTS Interpreting data. Calcium carbonate decomposes when heated to give calcium oxide and carbon dioxide.
EXPERIMENT 67 INVESTIGATE THE ACTION OF HEAT ON CARBONATE SALTS Conclusion. Does the law of conservation of mass hold true in this case. Li 2 CO 3 LiO 2 CO 2.
Bubbling carbon dioxide through this forms a milky suspension of calcium carbonate. Identify the carbonate salts that cannot be decomposed by heat. Enter your answer as a number g Element Relative atomic mass Ar- I 1 12 O 16 No 23 Ca 40.
Metal carbonates decompose when heated. Rest other carbonates decompose on heating. As lithium carbonate is not stable to heat it decomposes at lower temperature.
The decomposition of calcium carbonate. Heating a metal carbonate will decompose it into a metal oxide and liberate carbon dioxide. Use complete sentences to justify your answer based on numerical calculations.
All carbonates salts except potassium carbonate and sodium carbonate can be decomposed by heat to produce carbon dioxide gas. Small sized Li polarizes large carbonate ion which leads to formation of Li2O and CO2. This experiment can be carried out as a class exercise individually or in pairs.

Action Of Heat On Carbonate And Nitrate Salt

20 0 G Of A Magnesium Carbonate Sample Decomposes On Heating To Give Carbon Dioxide And 8 0 G Youtube
Lesson Explainer Thermal Decomposition Nagwa
Lesson Explainer Thermal Decomposition Nagwa

Calcium Carbonate Decomposes On Heating To Form Calcium Oxide And Carbon Dioxide When 10 G Of Youtube

The Carbonate That Will Not Decompose Heating Is
Lesson Explainer Thermal Decomposition Nagwa

Sodium Bicarbonate On Heating Decomposes To Form Sodium Carbonate Co 2 Youtube

Action Of Heat On Carbonate And Nitrate Salt
Action Of Heat On Salts A Plus Topper

Which Of The Following Carbonate Will Not Decompose On Heating Youtube

When Potassium Nitrate Is Heated It Decomposes Into Potassium Nitrite And Oxygen Write A Balanced Youtube

Ppt Access To He Diploma Powerpoint Presentation Free Download Id 2068254





Posting Komentar untuk "A Carbonate Not Decomposed By Heat"