Carbonate Buffer System
Rinse with thoroughly with plenty of water for at least 15 minutes. Rinse mouth with water.

Aquaponics Ph Explained How To Maintain The Perfect Balance Aquaponics Solid Shampoo Bar Well Water System
Nitric Acid - HNO.

Carbonate buffer system. Using optical traps to manipulate single DNA strands. Carbonate buffers Theory A classic buffer is a combination of a weak acid and its conjugate salt. Coating protein oon microplates.
Why is bicarbonate buffer system important. CarbBicarb Buffer pH 92 04. Well in any buffer system the boost in.
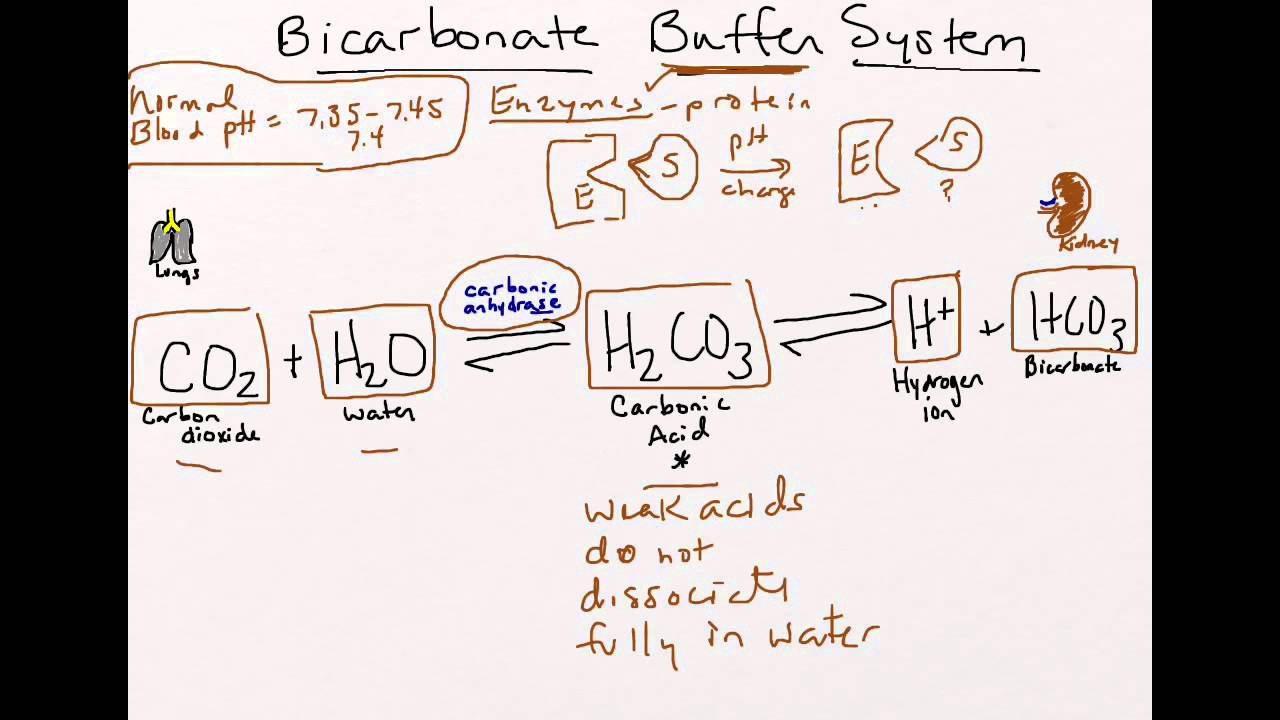
X ml of A y ml of B diluted to a total of 200 ml will yield the approximate pH shown. When the first proton is donated HCO3- otherwise known. Human blood contains a buffer of carbonic acid H 2 CO 3 and bicarbonate anion HCO 3 in order to maintain blood pH between 735 and 745 as a value higher than 78 alkalosis or lower than 68 acidosis can lead to death.
There are other uses but thats general knowledge of chemical solutions not a physiological phenomenon or system. If acid is added to this buffer the added H ions combine with bicarbonate ions to. Recall that buffers are mixtures of weak acids and their conjugate bases that resist changes in pH.
Add 9274 g of Sodium carbonate anhydrous to the solution. In this buffer hydronium and bicarbonate anion are in equilibrium with carbonic acid. Carbon dioxide plays a vital role in the chemistry of sea water.
Hydrochloric Acid Diluted Sodium Carbonate Citric Acid Monohydrate. Carbonatebicarbonate buffer Stock solution A. Typical biological buffers are the carbonic acid H 2 CO 3hydrogen carbonate HCO 3 which is present in blood plasma and saliva or the phosphate buffer H 2 PO 4 HPO 4 2.
With the sole exception of the chemistry of water itself this is by far the most complex equilibrium system and it touches upon every aspect of the Earth sciences-meteorology geology oceanography and. Oxalic Acid C. What happens when you titrate this combination with the strong acid of your choice.
Carbonic acid concentration is controlled by respiration that is through the lungs. The role of the bicarbonate buffer system in regulating blood pH This is the currently selected item. Describe how to prepare a sodium bicarbonate-carbonate buffer solution with a pH of 10.
The renal system can also adjust blood pH through the excretion of hydrogen ions H and the conservation of bicarbonate but this process takes hours to days to have an effect. If the pH of the blood decreases too far an increase in breathing removes CO 2 from the blood through the lungs driving the equilibrium reaction such that H 3 O is lowered. Its a buffer system that consists of a weak acid and its conjugate base.
He wrote an equation in 1908 to describe the carbonic acid-carbonate buffer system in blood. Hydrochloric Acid - HCl 0-2. A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3.
Calcium carbonate CaCO3 is a very common mineral. Henderson was broadly knowledgeable. Perchloric Acid HClO.
The carbonic acid - bicarbonate buffer system consists of carbonic acid a weak acid and the bicarbonate anion its conjugate base. One that is important in surface waters is the carbonic acidbicarbonate buffer. The carbonate buffer system plays an important role in regulating pH of physiological and environmental systems as it controls the exchange of carbon dioxide between the biosphere lithosphere atmosphere and aquatic systems Millero E 2020.
The carbonate and phosphate buffer systemsthese are not in the pH range necessary to buffer blood. The bicarbonate buffer system is what the body uses. Buffers in the pH.
In addition to his important research on the physiology of blood he also wrote. Potassium Chloride KCl 11-18. Carbonic acid is diprotic which means in has two H ions to donate to solution.
Seek medical attention if irritation occurs. The carbonate system encompasses virtually all of the environmental compartments the atmosphere hydrosphere biosphere and as CaCO 3 major parts of the lithosphere. The carbonic acid-hydrogen carbonate ion buffer works throughout the body to maintain the pH of blood plasma close to 74.
Remove to fresh air Ingestion. Bicarbonate buffer system is distinguished from non-bicarbonate buffers when analysing the titration of the latter by accumulating CO 2 by metabolic infl uences or during proton-equivalent ion exchange. 02 M solution of sodium hydrogen carbonate 168 g in 1000 ml For use.
The carbonate system which is the major source of bufiering in the ocean and is the main subject of this chapter. A buffer system has the property of resisting pH changes despite additions of acid or base. Buffer pKa and pH Range Values For preparation of.
In fact in addition to the regulating effects of the carbonate buffering system on the pH of blood the body uses breathing to regulate blood pH. The CarbonateBicarbonate Buffer System. 02 M solution of anhydrous sodium carbonate 212 g in 1000 ml Stock solution B.
If an accurate final pH is required titrate the two. Wash immediately with soap and water. Add 105 g of Sodium bicarbonate to the solution.
Add distilled water until volume is 1 L. 497-19-8 Sodium Carbonate Anhydrous Reagent Grade 08499A 1 Kg - MW. The basic relationship used to calculate pH of a buffer solution is the HendersonHasselbalch equation.
Carbonate Buffered solution 01M Sodium Bicarbonate-Sodium Carbonate Buffer pH 90 UPR16490 250 ml - Applications. The important thing to realize here is that carbonic acid H_2CO_3 is actually formed when carbon dioxide CO_2 is dissolved in water. 85 a system with a low buffer capacity should be used 3 so that when the ophthalmic solu-.
When atmospheric carbon dioxide is dissolved in seawater carbonic acid H 2 CO 3 is formed. One of the most important systems in the oceans is the CO2-carbonate system a buffering system that helps to maintain the pH of seawater to within a narrow range. He discovered that the acid-base balance in human blood is regulated by a buffer system formed by the dissolved carbon dioxide in blood.
For instance carbonic acid H 2CO 3 and sodium bicarbonate NaHCO 3 or even sodium bicarbonate and calcium carbonate. In natural systems there are many buffers. Sodium Carbonate Anhydrous ACS grade 141321 1 Kg 141322 25kg 141321 MW.
First aid measures Description of first aid measures. Buffers pKa range. The buffer systems functioning in blood plasma include plasma proteins phosphate and bicarbonate and carbonic acid buffers.
Carbonate-Bicarbonate Buffer pH 92 to 106 Preparation AAT Bioquest Inc 25 Oct. Changes in hydrogen carbonate ion concentration however require hours through the relatively slow elimination through the kidneys.

Coccolithophore Carbon Chemistry Carbon Cycle Diatom Ocean Acidification

Wet Grinding Process For Calcium Carbonate Calcium Carbonate Carbonate Calcium Carbonate Powder

Introduction To Full Frame Vs Crop Frame Sensors Plus Great Sensor Comparison Resources Full Frame Full Frame Vs Crop Full Frame Camera

8 58 10 80 Seachem Reef Builder 250gram Reef Builder Raises Carbonate Alkalinity Without Immediately Impacting On Ph Wit Pet Supplements Supplements Reef

Pin On Balcony Garden Plant Nutrients Hydroponic Gardening Plants

Hydroponics Farm Business Plan Sample Executive Summary Business Planning Farm Business How To Plan
Bicarbonate Buffer System System Study Tips Pharmacy School

Introduction To Rainwater Harvesting Rainwater Harvesting Rainwater Rain Barrels Diy

Specific Plant Ph Infographic Hydroponicsinfographic Hydroponicstips Hydroponicgardening Hydroponicsorgan Aquaponics System Aquaponic Gardening Hydroponics

Aquaponics Ph Part 3 Measuring Your Carbonate Buffer Aquaponics Aquaponics System Aquaponics Fish

Aquaponics Ph Explained Finding The Perfect Balance Aquaponics Balance Explained Finding Perfect Backyard Aquaponics Aquaponics Diy Aquaponics

General Hydroponics Ph Control Kit For A Balanced Nutrient Solution In 2021 Hydroponics Hydroponics Diy Gallon


Posting Komentar untuk "Carbonate Buffer System"