Carbonate Anion Test
Will conduct these several of these standard tests to confirm the presence of one of six possible anions in a three unknown solutions. Confirmatory anion testing is carried out using water extract when salt is water-soluble and using sodium carbonate extract when salt is water-insoluble.
Identifying Ions Noadswood Science Ppt Video Online Download
Add sodium sulfate solution to the test-tube until the tube is 13 full.

Carbonate anion test. The carbonate ion is the simplest oxocarbon anion. Testing for carbonate ions. When the salt is water-soluble confirmatory anion testing can be carried out using the water extract and when the salt becomes water-insoluble by using sodium carbonate extract.
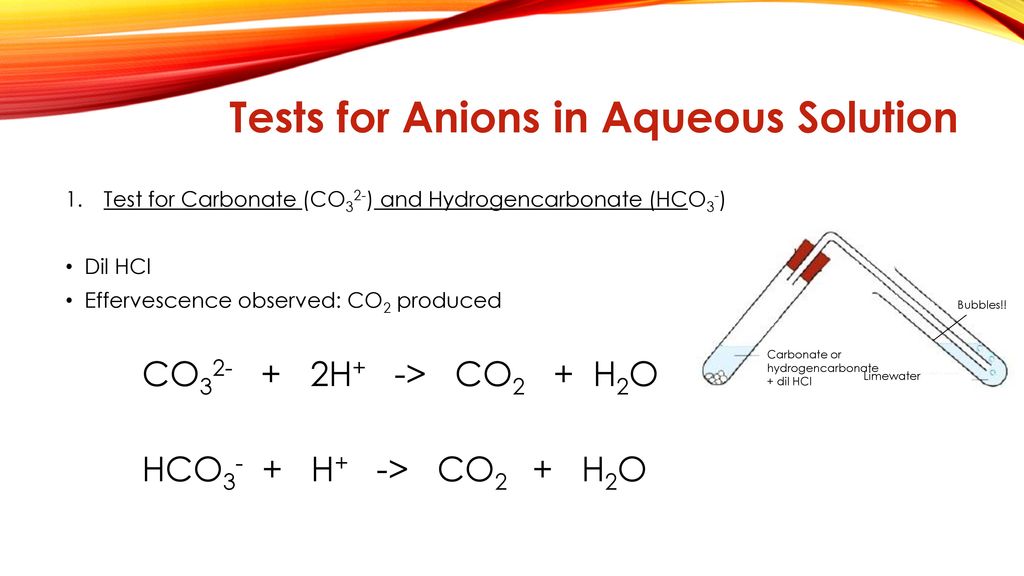
Carbonate CO 3 2- and Hydrogen Carbonate HCO 3 -1 Add 1 ml of sodium carbonate to a test tube add a few drops of HCl Repeat the experiment with aqueous potassium hydrogen carbonate Record the results in the table Add a few drops of Barium chloride 14. Formation of the gaseous CO 2 is a good indication of carbonate. Aqueous sodium carbonate and sulfuric acid react with the evolution of a.
To distinguish between carbonate ions and bicarbonate ions magnesium sulfate is added as a confirmatory test for carbonates and bicarbonates. Confirmatory Tests for S 2 CO 3 2- SO 3 2- CH 3 COO and NO 2 anions. The carbonate ion is the anion of carbonic acid which is a weak acid.
To test for Sulfate Ions. Reacts with dilute HCl to produce CO 2 gas. Carbonate Reactions CO3 2- A1.
Results HCO 3 -1 CO 3 2- Reaction with HCl followed by Barium chloride Anion 15. Some compounds of carbonate ion forms precipitates and some metal carbonates are soluble in water. Match each pair by selecting correct answer from the dropdown menu.
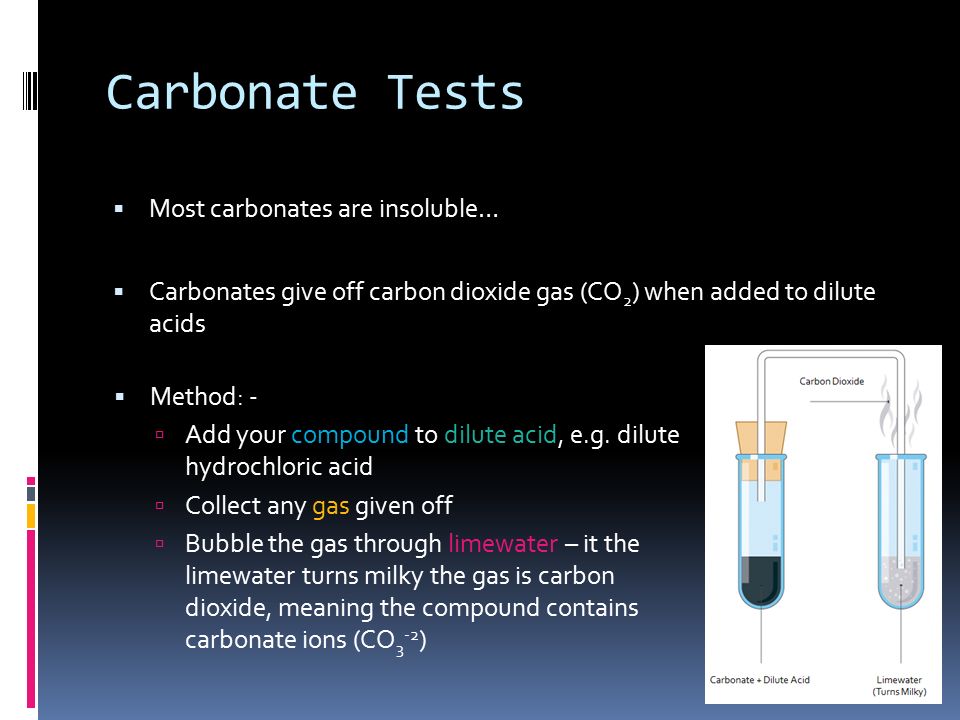
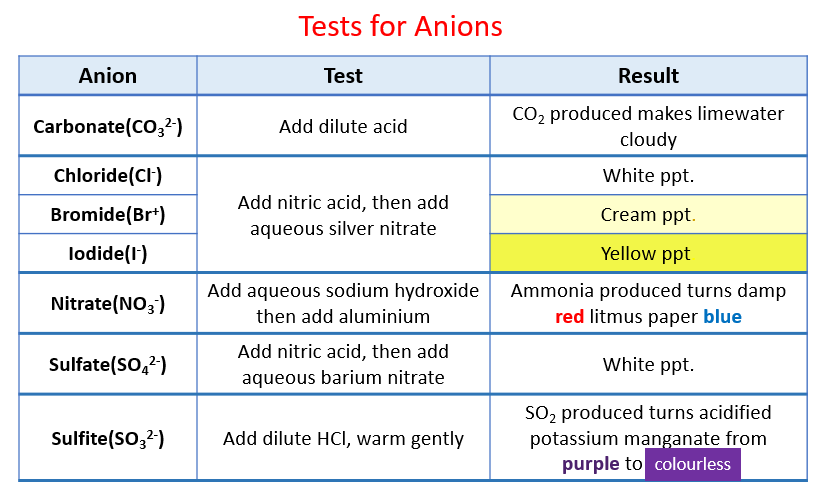
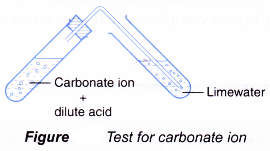
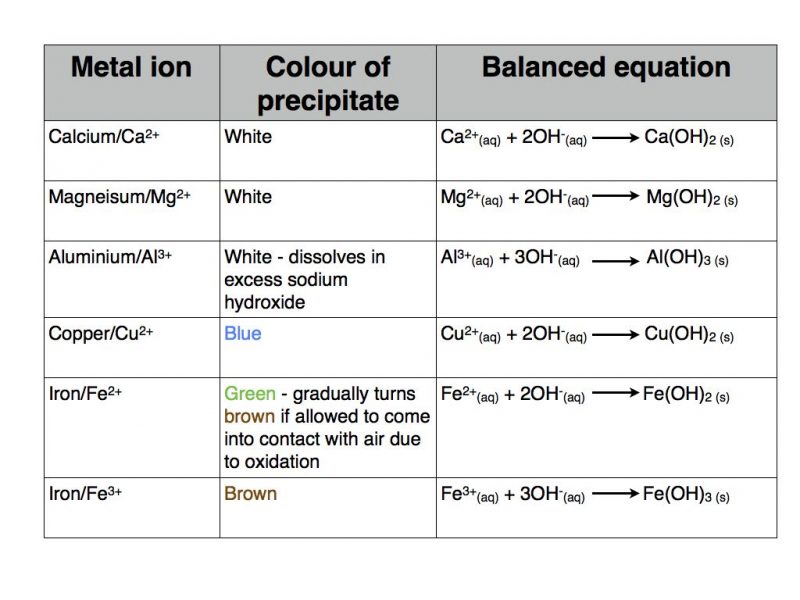
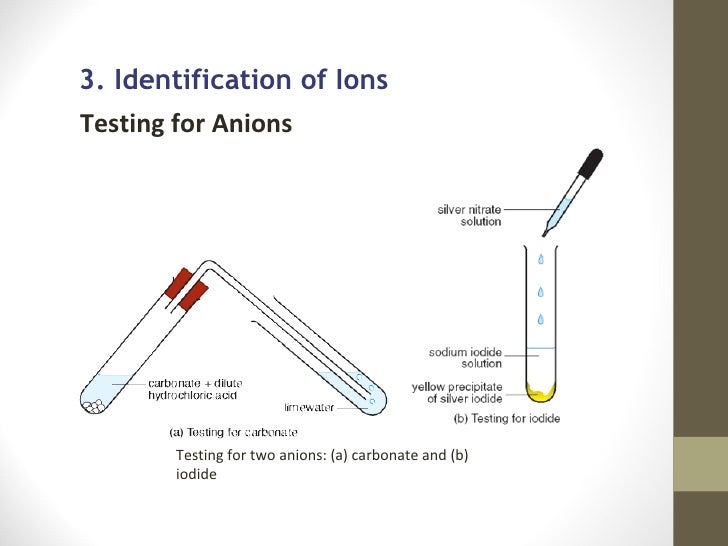
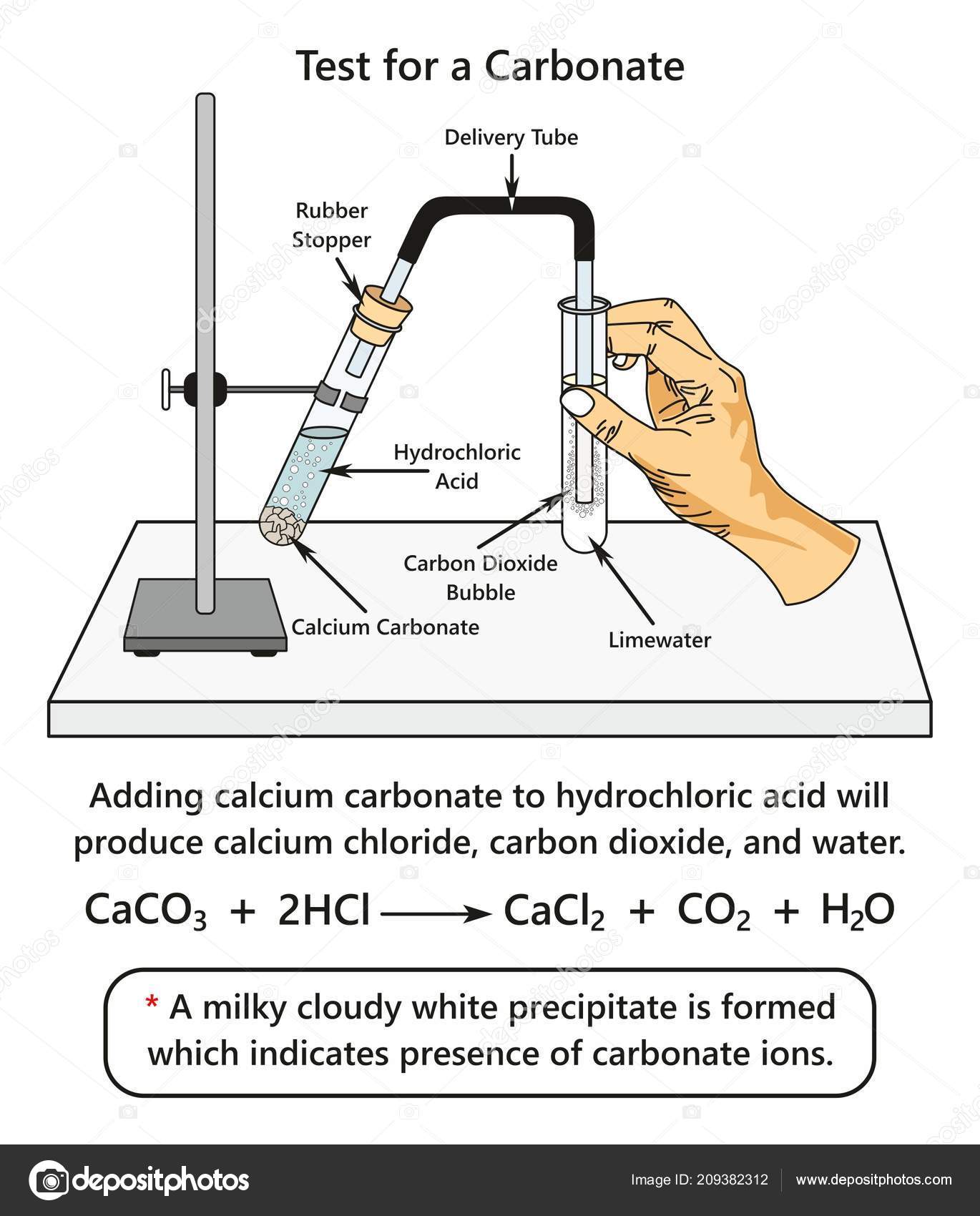
Confirmation of Carbonate CO 3 2- a Reaction with dil HCl. HCl gives CO 2 gas that reacts with lime water to produce a white precipitate of calcium carbonate that turns lime water milky. A summary of how each of these six anions reacts in these standard tests is provided below.
Carbonate on reaction with dil. It will produce a very weak fizz when a drop of. Put a spatula full of the carbonate into a test tube.
Some rocks contain carbonate minerals and the acid test can be used to help identify them. CO 3 2- 2 H CO 2 H 2 O. Aqueous sodium carbonate and sulfuric acid react with the evolution of a colorless odorless gas.
Dolostone is a rock composed of almost entirely of dolomite. Carbonate ions can be detected by treatment with dilute hydrochloric acid which produces carbon dioxide gas. Add dilute hydrochloric acid to the solid salt youre testing.
The sulfate ion is the anion of sulfuric acid H 2 SO 4 which is a strong acid but somewhat weaker than the hydrogen halide acids HCl HBr HI and nitric acid HNO 3. Remember when balancing Equations You may adjust the number in front of the atomsmolecules but you may not change the formula by changing the small number after an atom This method of balancing equations is known as balancing by inspection practice with question 61 a to n on p77 Tests for Anions in Aqueous Solutions Negative ions are also. How do you test for carbonate anion.
Ag Cl- AgCl. Testing for carbonate ions Carbonate ions CO 3 2 can be detected whether in a solid compound or in solution. It consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D 3h molecular symmetry.
The carbonate can be tested as a solid. Solutions containing hydrogencarbonate ions react with hydrochloric acid producing carbon dioxide gas. Limestone is composed almost entirely of calcite and will produce a vigorous fizz with a drop of hydrochloric acid.
Record your observations in the table b Theory Solutions containing hydrogencarbonate ions react with hydrochloric acid producing carbon dioxide gas. The test procedures for determining the identities of the unknown are based on the chemistry of these anions as shown by the following reactions. Na 2 SO 4 and K 2 SO 4 solutions are neutral and colourless.
Cl In this case CHE112 Cl-is detected by the preliminary test 1 only. Put 5cm3 limewater into another test tube. CO 3 2-To the 5-10 drops of the unknown add some acid HCl for example.
LIMESTONE DOLOSTONE AND MARBLE. A To test for the carbonate and hydrogencarbonate anions the step 4. Carbonate Identification Test Anion Salt Analysis - YouTube.
Tests for carbonate ion compounds reactions precipitates. For example NaHCO 3 HCl NaCl H 2O CO 2 Solutions containing carbonate ions also react with hydrochloric acid. Anion Tests Matching Quiz.
Sulfate ion exists as many compounds. Add 5cm3 dilute hydrochloric acid to the carbonate. Also some metal carbonates have colours in solid state and aqueous state.
Then click the Check button to see your result. In case of soluble carbonate this test is. Wet Tests for Acid Radicals Anions Let us discuss the chemical reactions involved in the confirmation of Anions.
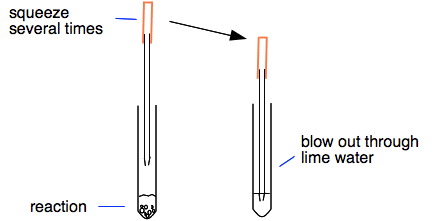
Carbon dioxide gas bubbles if carbonate ions are present. Test any gas that forms by bubbling it through limewater. Limewater is used to confirm that the gas is carbon dioxide.
Confirmation of CO32is done using aqueous salt solution or using solid salt as such as carbonate ions are contained in the sodium carbonate extract. For example NaHCO3 HCl NaCl H2O CO2 Solutions containing carbonate ions also react with hydrochloric acid producing carbon dioxide gas. The Acid Test on Rocks.
SO 4 2-To the 5-10 drops of the unknown add 1-2 mL of HCl solution and some BaCl 2Formation of the white precipitate. Testing for sulfate ion Qualitative Analysis for SO. To test for the carbonate and hydrogencarbonate anions.
It has a molecular mass of 6001 gmol and carries a total formal charge of 2. It is the conjugate base of the hydrogencarbonate bicarbonate ion HCO. Quickly put the bung of the delivery tube in the test tube with the other end in the lime water.
This gas can be passed through limewater turning it cloudy. When added to MgSO 4 solution it produces a white precipitate. Carbonate nitrate nitrite phosphate sulfate and sulfite.
Add barium chloride solution drop wise. In this tutorial we study carbonate ion. An acid such as dilute hydrochloric acid is added to the test compound.
Lesson Explainer Tests For Anions Nagwa
Identify Anions Solutions Examples Activities Experiment Videos

Qualitative Analysis Tests For Anions Identifying Negative Ions Hydroxide Alkalis Identification
Test For Cations And Anions In Aqueous Solutions A Plus Topper
Lesson Explainer Tests For Anions Nagwa
6 Chemical Equations Tests For Anions Ppt Download
Chemical Tests Gcse Chemistry Online Chemistry Tutor
E Learning Identification Of Ions And Gases
45 Adding Salt Vector Images Adding Salt Illustrations Depositphotos
Lesson Explainer Further Tests For Anions Nagwa

Tests For Anions Definition Examples Diagrams

Tests For Ions Sunday 11 November Ppt Download

Identifying Anions Spm Chemistry



Posting Komentar untuk "Carbonate Anion Test"