Carbonate Lewis Structure Resonance
Total number of electrons of the valance shells of CO 3 2-Carbon is located at group 4 in the periodic table. Lets do the CO3 2- Lewis structure.
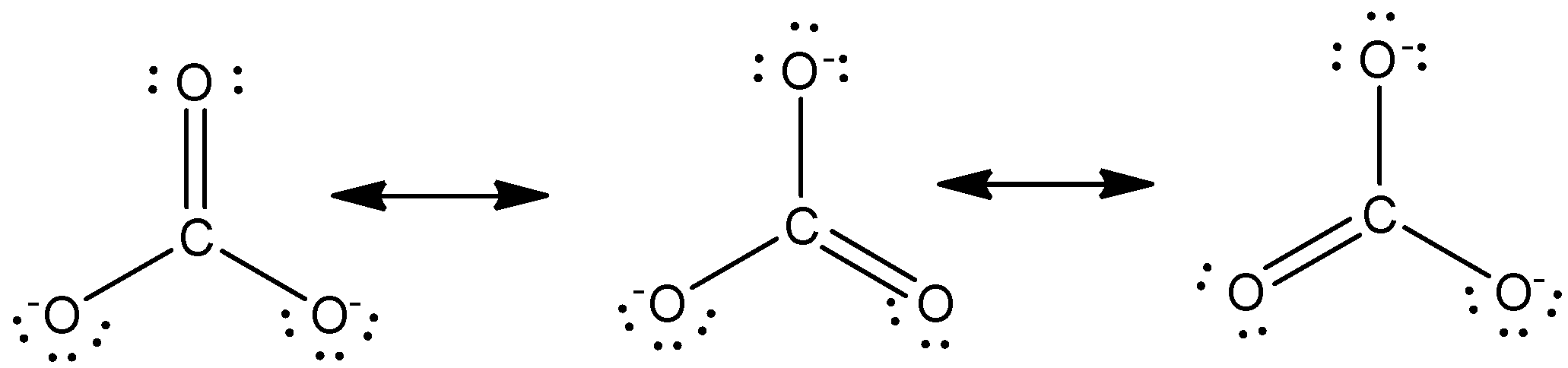
Write The Resonance Structure Of Carbonate Ions Class 12 Chemistry Cbse
Carbonate ion a moderately strong base undergoes considerable hydrolysis in aqueous solution.

Carbonate lewis structure resonance. Determine the formal charge of each atom. The actual electronic structure of the molecule the average of the resonance forms is called a resonance hybrid of the individual resonance forms. Instead we use the concept of resonance.
The Carbonate CO23 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. 91 470 ratings FREE Expert Solution. The Carbonate CO_32 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu. A double-headed arrow between Lewis structures indicates that they are resonance forms. CO2 CO3 2 NO3 O3.
The CO32-ion contains one C-0 single bond and two C-O double bonds. Figure 5 Carbonate Ion as a Resonance Hybrid. These three resonance structures.
When it is possible to write more than one equivalent resonance structure for a molecule or ion the actual structure is the average of the resonance structures. The actual distribution of electrons in each of the nitrogen-oxygen bonds in textNO_2text is the average. Similarly what is the Lewis structure for co3 2.
Each carbon oxygen bond can be thought of as 1333 bonds. It has six electrons in valence shell. Is CO3 2 an acid or base.
How many Lewis structures does CO3 2 have. Transcribed image text. Learn this topic by watching Resonance Structures Concept Videos.
Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Whenever there are two or more valid Lewis structures for the same compound the true structure of the molecule is best represented as a hybrid of the individual structures. If two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion the actual distribution of electrons is an average of that shown by the various Lewis structures.
Draw the Lewis structure of the carbonate ion CO 3 2- showing all possible resonance structures if there are any. A covalent compound is said to exhibit resonance if we can draw two or more valid Lewis structures that describe the bonding in its molecule. There are three different possible resonance structures from carbonate.
Carbonate ion resonance structure of carbonate ion CO32 CO32 molecule ion drawn of SO 3 2-ion this ion has one carbonoxygen double bond lone. Draw the Lewis structure for CO32- including any valid resonance structures. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures.
In this regard how many Lewis structures does co32. So carbon has four electrons in its valence shellOxygen is located at 6 th group. The Carbonate C O 3 2 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
The CO32- ion contains two single bonds and one C_0 triple bond. Lewis structure - resonance carbonate Polyatomic ion whose formula is HCO3- trigonal planar. I also go over the resonance hybridization shape and bond angle.
Figure 5 presents the resonance hybridthat results from mixing the resonance contributors1 2 and 3shown in Figure 2. Draw the Lewis structure of the carbonate ion CO32- showing all possible resonance structures if there are any. Three resonance structuresLike ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure Unlike O3 though the actual structure of CO32 is an average of three resonance structures.
Determine the formal charge of each atom. One may also ask is co3 2 a resonance structure. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
The carbonate anion CO 32 provides a second example of resonance. The average of a double bond and 2 single bonds. Unlike O3 though the actual structure of CO32 is an average of three resonance structures.
Drawing correct lewis structure is important to draw resonance structures of CO 3 2-correctly. How many resonance structures does co32. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
Because carbon is the least electronegative element we place it in the central position. Describe one resonance structure of the carbonate ion.
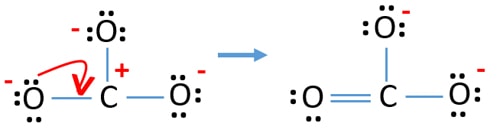
Lewis Structure For Co32 Carbonate Ion

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com
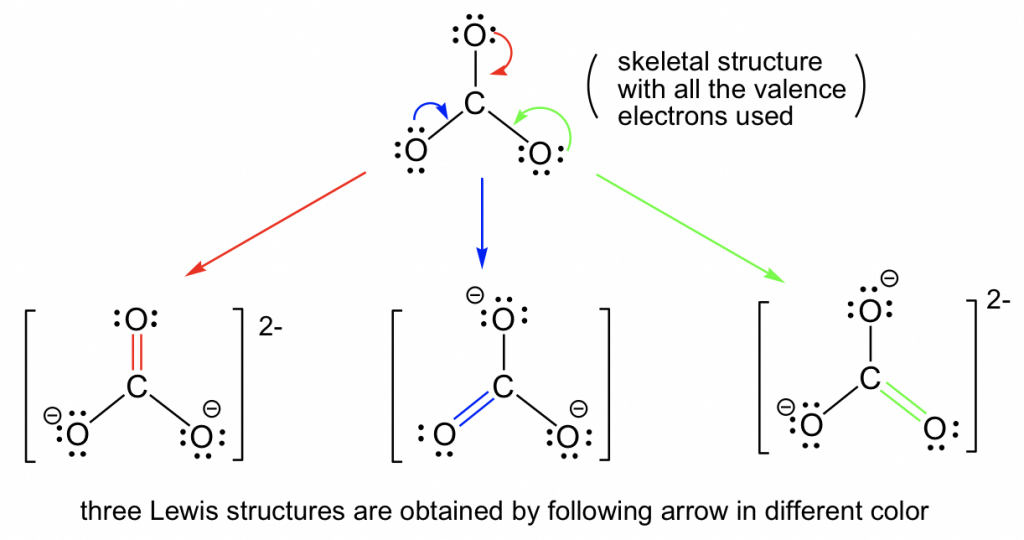
1 3 Resonance Structures Organic Chemistry
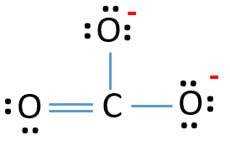
Lewis Structure For Co32 Carbonate Ion

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

What Is Carbonate S Lewis Structure Study Com

Draw And Explain The Lewis Dot Structure Of Co32 Include All Lone Pairs Of Electrons Study Com

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Resonance Structures For Co3 2 Carbonate Ion Youtube
Lewis Structure For Co32 Carbonate Ion
What Is The Lewis Structure Of Co3 2 Quora
Posting Komentar untuk "Carbonate Lewis Structure Resonance"