Carbonate Anion Lewis Structure Lone Pairs
4 3 7. The shape of the carbonate anion is not determined by the total number of electron pairs rather the number of places where electrons can be found.
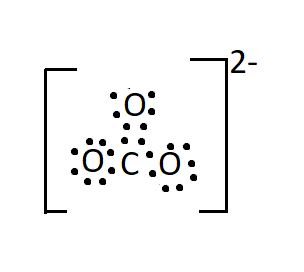
Draw The Lewis Structure For Carbonate Ion Co32 Class 12 Chemistry Cbse
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells.

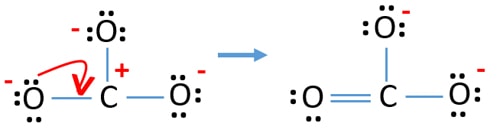
Carbonate anion lewis structure lone pairs. Replace two lone pairs with one bond. Assign lone pairs radical electrons and atomic charges where appropriate check_circle. Divide the number of electrons by two to give the number of electron pairs.
They are a simplified version of the molecular geometry of a compound and can be used to depict the chemical bonding in the molecules. 12 electron pairs involved in bonding or present as lone pairs. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells Total electron pairs are determined by dividing the number total valence electrons by two.
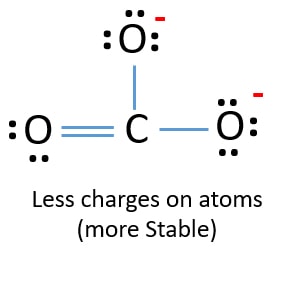
One can draw Lewis structures to see where the lone pairs of electrons may be. Add 3 electrons for the three σ-bonds. Oxygen should hold negative charges in the lewis structure because oxygen likes to keep electrons than carbon atom.
7 1 6. It is the simplest oxocarbon anion consisting of one carbon atom and two oxygen atoms. Ask your question and find the answer free.
For OH-ion we use those steps. The shape of the carbonate anion is not determined by the total number of electron pairs rather the number of places where electrons can be found. There are several steps to draw a lewis structure.
Nitrogen tends to form three bonds and have on e lone pair. What is the Lewis structure of the polyatomic anion IF 4. Also there are no charges in those oxygen atom.
Count the electrons you have. Here you can see that you can draw the ion like this with one of the oxygen atoms having a double bond with the carbon and the other two having a single bond. Those steps are explained in detail in this tutorial.
Steps of drawing lewis structure of OH-ion. ClO2- lewis structure contains one single bond and one double bond. Questions asked by students.
The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. There is only one sigma bond between hydrogen and oxygen atoms in lewis structure of OH-ion. A Lewis structure or Lewis representation also known as electron raster diagram Lewis raster formula Lewis point structure or point electron structure is a two-dimensional diagram used in chemistry to show the bonding between atoms of a molecule and the lone electron pairs that may be present in this molecule.
It will share all 4 valence electrons through a double covalent bond with one peripheral oxygen atom and 2 single bonds. Oxygen tends to form two bonds and have two lone pairs. If there are too many replace two electron pairs with a single bond until you have the correct number of valence electons.
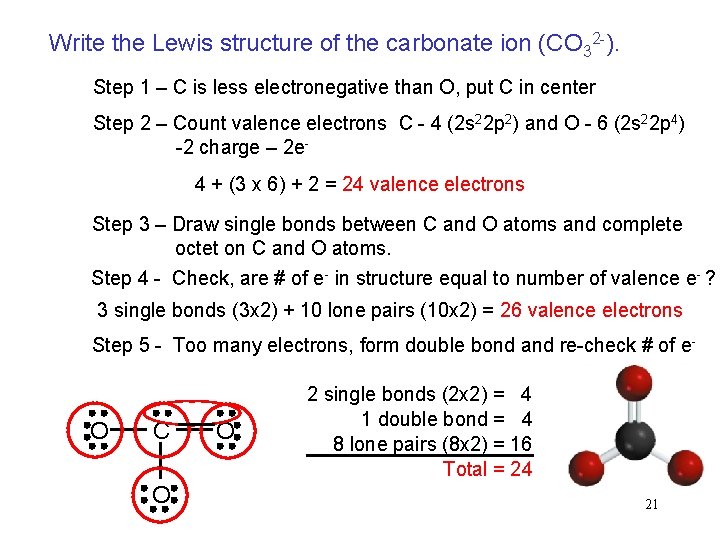
Check the stability and minimize charges on atoms to build the most stable structure by converting lone pairs to bonds. Formally the 1s2 electrons and for OC-O-_2 there 24 electrons ie. 3 bonds x 2 6 10 lone pairs x 2 20 Total is 26.
Total electron pairs are determined by dividing the number total valence electrons by two. Draw the Lewis structure for carbonate ion CO2 3 CO 3 2. Therefore In the Lewis structure of I C l 2 3 lone pairs of electrons are around the iodine atom.
Those two oxygen atom are connected to the carbon atoms by a double bond has two lone pairs in its last shell. The relative atomic mass has no units. And two single bonds between carbon and two negatively charged oxygen atoms.
Carbon has 4 valence electrons. Like these steps following facts are important to draw the lewis structure. Carbon C contains 4 valence electrons on its own as main group 4A element.
Seeing the structure one would imagine that in carbonate ion two bonds will be the same in length the two single bonds and will be longer as compared to the third bond. Subtract one electron for each π-bond. Put lone pairs on atoms.
Carbon tends to form 4 bonds and have no lone pairs. To draw the lewis diagram of any molecule we have to follow 5 or 6 simple steps depending on the complexity of the molecule. The ion has a trigonal planar arrangement with a molecular mass of 60 u.
The valence electrons of the atoms of a molecule can be represented using the Lewis dot structure. The Lewis dot structures of the individual non-metal atoms give a good indication of the bonding possibilities for the atoms. Lewis diagram is a representation of the valence electron within a molecule.
Bicarbonate also known as hydrogen carbonate is formed when carbonic acid is deprotonated. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. The Lewis structure of the anion has one double bond between a neutral oxygen atom and carbon.
On carbon atoms there are no lone pairs or a charges. For IO 4 - there are 32 valence electrons pairs so total pairs of electrons are 16. The calculation of relative formula mass is done the same as the calculation of molecular weight where atomic mass of the atoms are added but the unit of molecular weight is gmol.
Two lone pairs present on the central atom of the ClO2- Lewis structure. Two bonding pairs and no lone pairs linear geometry. Carbonate ion is a polyatomic ion.
There are two another oxygen atoms. 4 bonds x 2 8 8 lone pairs x 2 16 Total is 24. Exercise PageIndex2 What is the shape of H 2 CO.
And their presence is clear from the Lewis structure of carbonate anionthere are 8 inner core electrons ie. Also there are three lone pairs on oxygen atom.
Lewis Structure For Co32 Carbonate Ion

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
Lewis Structure For Co32 Carbonate Ion
Chemical Bonding I Basic Concepts Chapter 9 Copyright
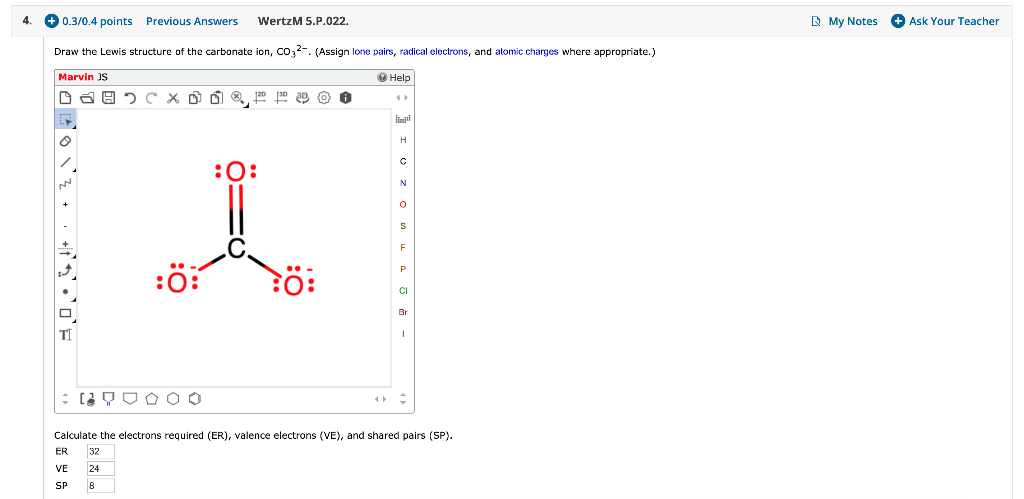
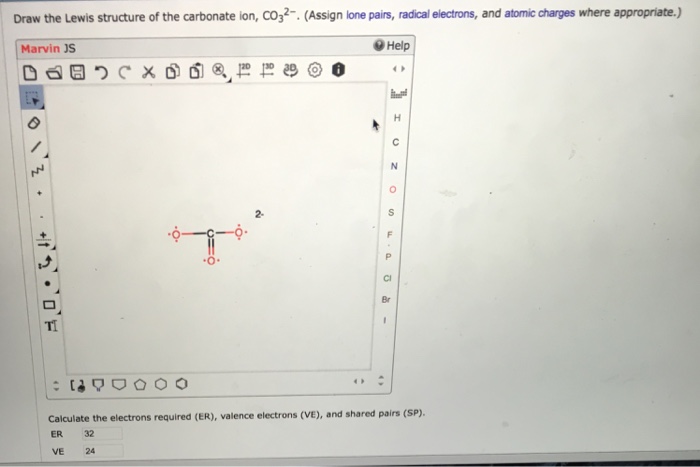
Solved Draw The Lewis Structure Of The Carbonate Ion Co32 Chegg Com
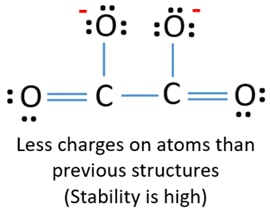
Lewis Structure For C2o42 Oxalate Ion
What Is The Lewis Structure Of Co3 2 Quora
Solved Draw The Lewis Structure Of The Carbonate Ion Chegg Com

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw A Lewis Structure For The Hydrogen Carbonate Ion Including Lone Pairs And Formal Charges Study Com
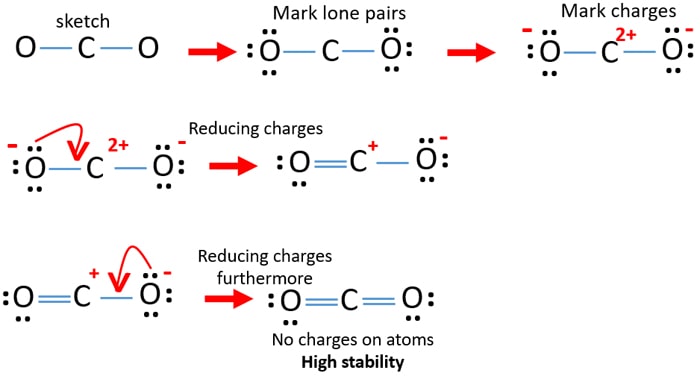
Co2 Carbon Dioxide Lewis Structure And Shape

Calcium Carbonate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic
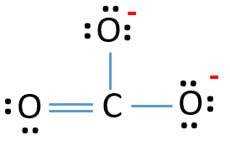
Lewis Structure For Co32 Carbonate Ion
Posting Komentar untuk "Carbonate Anion Lewis Structure Lone Pairs"