Carbonate 2 Lewis Structure
Lewis Structure of O 2. Lets do the CO3 2- Lewis structure.
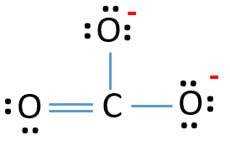
Lewis Structure For Co32 Carbonate Ion
4 plus 18 plus 2.

Carbonate 2 lewis structure. Carbon is the least electronegative put that at the center. The carbonate ion is the simplest oxocarbon anion consisting of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement. Hence in the Lewis structure the C atom is the central atom.
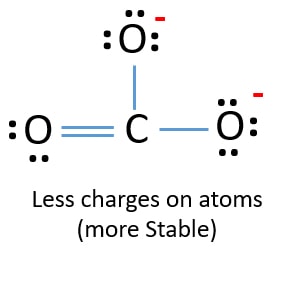
Therefore it is doubly bonded to each oxygen atom. Carbon contains four valence electrons resulting in zero lone pairs. The Lewis structure of the carbonate ion has two long single bonds to negative oxygen atoms and one short double bond to a neutral oxygen.
NCI Thesaurus NCIt Carbonate is a carbon oxoanion. The ion has a trigonal planar arrangement with a molecular mass of 60 u. Draw the Lewis structure of the carbonate ion CO 3 2.
For the CO32- Lewis structure the total number of valence electrons found on the periodic table for the CO32- molecule. The Lewis structure of the carbonate ion has two single bonds to negative oxygen atoms and one short double bond to a neutral oxygen. I am not considering the resonance that will take place in the carbonate ion.
Two carbon atoms are joint to one carbon atom. A step-by-step explanation of how to draw the CO32- Lewis Structure Carbonate Ion. 4 plus 18 plus 2.
Draw the Lewis structure for carbonate ion CO2 3 CO 3 2. Now we are going to learn how to draw this lewis structure. Carbonate has the formula CO 3.
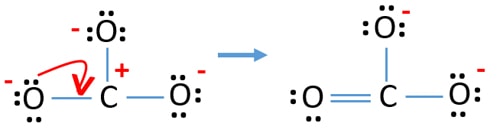
This structure is incompatible with the observed symmetry of the ion which implies that the three bonds are equally long and that the three oxygen atoms are equivalent. CO32- Lewis Structure - How to Draw the Lewis Structure for CO3 2- Carbonate Ion - YouTube. It is the simplest oxocarbon anion consisting of one carbon atom and two oxygen atoms.
Polyatomic ions are composed of atoms that share electrons but they have excess electrons that give them an electric charge. There are three different possible resonance structures from carbonate. In the CO32- Lewis structure carbon is the least electronnegative element.
Oxalate ion C 2 O 4 2-Carbonate ion has a -2 charge. Add that all up. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
There are two carbon atoms in the oxalate ion. How many electrons are in a carbonate ion. Carbonate ion is a polyatomic ion.
Oxygen has six we have 3 Oxygens and this negative 2 means we have an extra two valence electrons. This is the lewis structure. Carbon is the least electronegative put that at the center.
How to draw the Lewis Dot Structure for Calcium Carbonate - YouTube. Three Oxygens go around the Carbon. C 2 O 4 2-Lewis structure.
Carbonate lewis structure and bicarbonate lewis structure In carbonate ion there is two oxygen atoms which has -1 charge on each of them. CO3 2- is carbonatea carbonate is a salt of carbonic acid H2CO3characterized by the presence of the carbonate ion a polyatomic ion with the formula of CO3 2-. 70 More Lewis Dot Structures.
The Carbonate ion is used frequently in chemistry and worth spending time to fully understand. Lewis structure of NO 2-ion is drawn in this tutorial. The number of valence electrons in C and O.
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. It is a conjugate base of a hydrogencarbonate. Steps of drawing lewis structure of C 2 O 4 2-.
Oxygen has six we have 3 Oxygens and this negative 2 means we have an extra two valence electrons. Lets do the CO3 2- Lewis structure. Assign lone pairs radical electrons and atomic charges where appropriate Calculate the electrons required ER valence electrons VE and shared pairs SP.
One of these oxygen atom take a proton H ion and form a -OH group. This video discusses the resonance structu. The central atom of this molecule is carbon.
Carbon has 4 valence electrons. Answer 1 of 2. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-.
Carbonates are readily decomposed by acids. In CO2 3 C O 3 2 C is the least electronegative atom. Since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule.
As like that other two oxygen atoms has joint to other carbon. Since it is bonded to only one carbon atom it must form a double bond. In the lewis structure of C 2 O 4 2-ion ion is symmetrical around the C-C bond.
Oxygen contains 6 valence electrons which form 2 lone pairs. Lewis Structure for NO 2-Nitrite ion. Carbon has 4 valence electrons.
Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure. Metal carbonates generally decompose on heating liberating carbon dioxide and leaving behind an oxide of the metal. Lewis Structure of CO 2.
Add that all up.
Lewis Structure For Co32 Carbonate Ion
Lewis Structure For Co32 Carbonate Ion
Download 35 Possible Resonance Structures For Co32

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

What Is Carbonate S Lewis Structure Study Com

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

In The Carbonate Anion Which Atoms Gain The Two Electrons Chemistry Stack Exchange
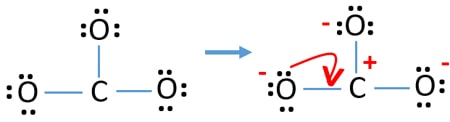
Lewis Structure For Co32 Carbonate Ion

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
What Is The Lewis Structure Of Co3 2 Quora

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Draw Lewis Dot Structure For Co3 2 Carbonate Ion Youtube
Posting Komentar untuk "Carbonate 2 Lewis Structure"