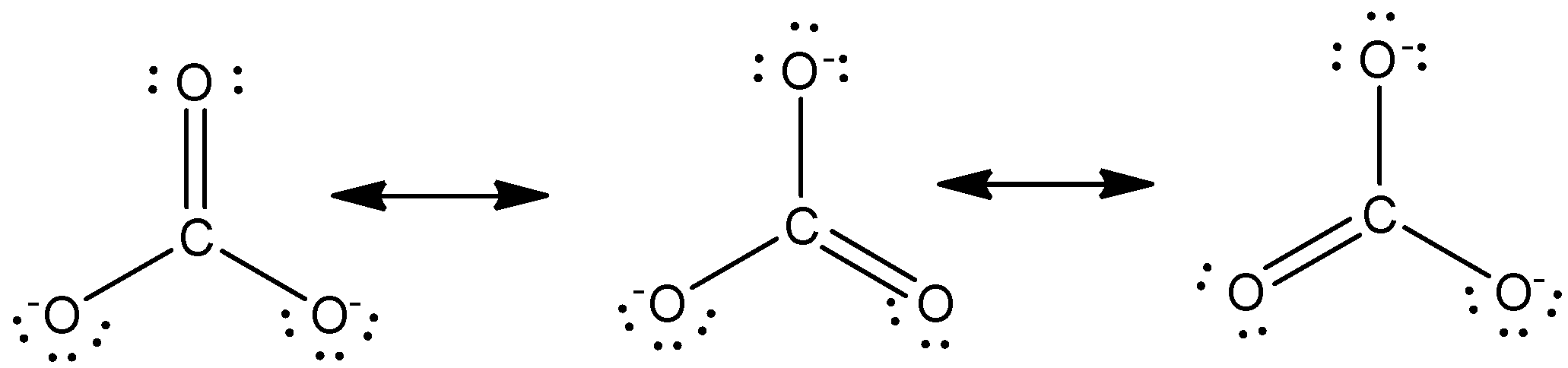
Carbonate Anion Resonance Structures
Consider all equivalent resonance structures for carbonate anion shown below. That means there is one C-O.

Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 Molecular Geometry Writing Chemistry
Consider all equivalent resonance structures for the carbonate anion shown below.

Carbonate anion resonance structures. The bond length is intermediate between a. Normally the number of bonds between two atoms in the Lewis structure can tell you how closely the two atoms are held. However studies of the electron density and bond length in the nitrate V ion indicate all the bonds are equal in length and the electron density is spread evenly between the three oxygen atoms.
This problem has been solved. A resonance structure means that there are more than one way to draw the ion. Carbonate ion a moderately strong base undergoes considerable hydrolysis in aqueous solution.
17 In consequence of the protonation the symmetry changes to approximately C s symmetry with dihedral angles of 17562 O1C1O3C3 and 17683 O1C1O2C2. All oxygen atoms however are equivalent and the double bond could form from any one of the three atoms. Drawing correct lewis structure is important to draw resonance structures of CO 3 2-correctly.
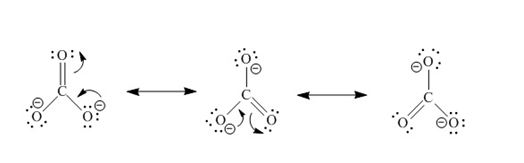
What is the formal charge on O1. In general resonance occurs when we can delocalize a non-bonding valence electron pair through 3 or more adjacent atoms while maintaining the same connected order of atoms. These structures are called resonance structures.
This gives rise to three resonance forms of the carbonate ion. Be sure to include all resonance structures that satisfy the Question. One oxygen atom must have a double bond to carbon to complete the octet on the central atom.
Draw the Lewis structure for the polyatomic carbonate COj anion. All oxygen atoms however are equivalent and the double bond could form from any one of the three atoms. The Carbonate C O 3 2 Ion.
The recently reported crystal structure of ethylene carbonate shows C 2 symmetry with CH 2 groups deviating out of plane. One oxygen atom must have a double bond to carbon to complete the octet on the central atom. The answer derived here is applicable to 3 different resonance structures of the carbonate anion.
We start with a valid Lewis structure and then follow these general rules- Resonance. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. One oxygen atom must have a double bond to carbon to complete the octet on the central atom.
They appear very similar but the formal charges are moved around through the delocalization of pi. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. 1 Carbon - 4 3 Oxygen - 63 18 And the charge of -2 gives you an additional 2 electrons.
Consider the following equivalent resonance structures for the carbonate anion. Lets consider the example of carbonate anion CO 3 2-. Resonance structures can be either equivalent or non-equivalent.
This gives rise to three resonance forms of the carbonate ion. There are equivalent three resonance structures CO32- the nitrite ion. Consider the following equivalent resonance structures for the carbonate anion.
What is the average charge on each oxygen atom. If the bond order is defined as the number of electron pairs shared between two atoms. Consider the following equivalent resonance structures for the carbonate anion.
Unlike O 3 though the actual structure of CO 32 is an average of three resonance structures. 211112121 12 bonds A. The need for resonance structures.
The carbonate anion ceCO32- provides a second example of resonance. Total number of electrons of the valance shells of CO 3 2-Carbon is located at group 4 in the periodic table. The carbonate anion shown below has one double bond and two single bonds.
So carbon has four electrons in its valence shellOxygen is located at 6. Charges have not been shown. The carbonate anion CO 3 2 CO 3 2 provides a second example of resonance.
This is a carbonate ion. Asked Jun 15 2017 in Chemistry by tattoo_guy58. Interestingly the CO 3 moiety is absolutely planar.
Is CO3 2 an acid or base. The carbonate anion CO 3 2 provides a second example of a polyatomic ion with equivalent resonance or equally weighted resonance structures. Charges have not been shown.
Resonance Structures of a Polyatomic Ion - Carbonate CO32- - YouTube. First determine the total number of electrons available. What is the average bond order for the C-O bond Look on TopHat Bond orders of 9 bonds.
Negative charges have not been shown. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. 13 Resonance Structures In the case that more than one reasonable plausible Lewis structure can be drawn for a species these structures are called resonance structures or resonance contributors.
30 0 o А -013 В -067 O D 013 E 067 Unanswered 2 attempts left Sube.

Consider The Resonance Structures For The Carbonate Ion Image Src Charge5986180229036662068 Jpg Alt Charge Caption Study Com

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion In 2021 Molecular Geometry Molecular Geometry
Write The Resonance Structures Of Co32 And Hco3 Scholr
Write The Resonance Structure Of Carbonate Ions Class 12 Chemistry Cbse

Chemistry Net Simple Procedure For Writing Lewis Structures Of A Chemistry Lewis Writing
Solved Consider The Resonance Structures For The Carbonate Ion A How Much Negative Charge Is On Each Oxygen Of The Carbonate Ion B What Is The Bond Order Of Each Carbon Oxygen Bond In

Valence Bond Structure Of Carbonate Ion Co3 2 Download Scientific Diagram

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Exp Chemistry Classroom Chemistry Lessons Teaching Chemistry
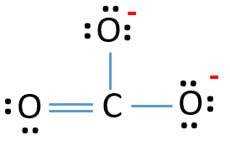
Lewis Structure For Co32 Carbonate Ion

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube

Lewis Dot Structure Of Co3 2 Carbonate Ion Youtube Goes Over Resonance As Well Dots Carbonate Lewis

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube

Chemistry Net Simple Procedure For Writing Lewis Structures Ex Writing Pi Bond Octet Rule
Posting Komentar untuk "Carbonate Anion Resonance Structures"