Carbonate Ion Bond Angle
H 2 g θH g H g. Like the sulphate ion the negative charges are again found on two of the oxygen atoms.

Valence Shell Electron Pair Repulsion Theory Vsepr
The VSEPR theory therefore predicts that CO 2 will be a linear molecule just like BeF 2 with a bond angle of 180 o.

Carbonate ion bond angle. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. C there are three electronic regions around carbon. HCONH20-C-N 12470 resonance HCONH C-NH 120 and 11859 structure 1 HCONH.
Now we are going to learn how to draw this lewis structure. The two single bonds and the double bond unit arrange themselves as far apart in a trigonal planar arrangement as possible - precisely similar to the carbonate ion which is given below. Energy required to break one mole of a particular type of bond between two atoms in gaseous state Example.
Bond order 3 4 numberlocations numberbonds Bond angle is 120º Molecular geometry is trigonal planar Please draw structures possibly starting with Lewis Structures and answer the following questions. The O-N-O bond angle in the nitrate ion is. The central carbon atom in the carbonate CO32- ion is sp2 hybridised.
HCONH0-C-N Same as 1247 resonance HCONH C. Applicable indicate the molecular Shapes anc bond angles indicate the molecular polarity if any. It is a conjugate base of a hydrogencarbonate.
D With two nuclei around the central atom and one lone pair of electrons the molecular geometry of SnCl 2 is bent like SO 2 but with a ClSnCl bond angle of 95. For example water should have bond angles of 1095 degrees but in reality its around 1045 or something like that. The two C-O single bonds and the CO double bond.
Lewis structure of NO 2-ion is drawn in this tutorial. So two of those pairs form a double bond. One of the 3 oxygens is enaged in the double bondand the other two oxygens connected to craboon by a single sigma bond each hold the negative charge of one electron excess each.
Δ a H 4358 kJ mol 1 O 2 OO g θO g O g. The carbon atom does not have an octet b. It has a Trigonal Planar geometry.
Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure. What is the exact bond angle of the carbonate ion. Total electron pairs are determined by dividing the number total valence electrons by two.
E molecular geometry is trigonal pyramidal. For CO 3 2-ion Total pairs of electrons are 12. As shown above the CO 3 2-ion has symmetrical nature.
If playback doesnt begin shortly try restarting your device. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. Molecule bond VSEPR- WebMO Do both models Experimentally- angle of interest predicted calculated predict the same determined bond angle bond angle angle within 392 bond angles CH3NH2C-N-H 111 HCONH.
Therefore its polar bonds are distributed evenly. Carbonates are readily decomposed by acids. This results in equivalent bond angles of 120 making the structure symmetrical in nature.
The ion is planar with a bond angle of 120 0. Carbonate Ion is a polyatomic ion with formula of CO3 2-. The carbon in Carbonate ion is in an sp2 hybridisation and there are 3 sigma bonds and one pi bond around it.
What is the bond angle in the carbonate ion CO32-. What bond angle is most closely associated with a linear distribution of electron density. D All the bonds have the same length and strength.
Carbonate ion CO 3 2-is trigonal planar in shape with a O-C-O bond angle of 120 o because of three groups of bonding electrons and no lone pairs of electrons. To be the center atom ability of having higher valance is important. The ion has 22 valence electrons.
A the bond is stronger than C-O and weaker than CO. Image will be Uploaded Soon State of Hybridization of N In. And identify the major intermolecular force in each compound Hint In this worksheet as In all chemgstry problems youll see polyatomic Ions arent drawn as big Of atoms carbon tetraflucride 3 5 H2CS carbonate ion Cavae pbshtog.
But these electrons are concentrated in three places. Net Dipole Moment and 2- Charge. The carbonate ion CO 3 2-The carbonate ion is bonded like this.
28 Which one of the following statements is incorrect about the carbonate ion. The two double bond units and the two single bonds arrange themselves as far apart as possible to give a tetrahedralarrangement. Which of the following is true in the carbonate ion CO32-.
Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. The shape is deduced below using dot and cross diagrams and VSEPR theory and illustrated below. Now the original lone pair has become a bonding pair.
The molecular geometry can be described as a trigonal planar arrangement with. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. Δ a H 498 kJ mol 1 N 2 NN g N.
Angle between the bonds around the central atom in a moleculecomplex ion Bond Enthalpy Δ bond H. Lewis Structure for NO 2-Nitrite ion. Because the lone pair of electrons occupies more space than the bonding pairs we expect a decrease in the ClSnCl bond angle due to increased LPBP repulsions.
Center atom of CO 3 2-ion. B the bond angle is 120. The molecular geometry of the nitrate ion is.
I2 Iodine Gas Molecular Geometry Bond Angles Electron Geometry. Theoretically it should be 120 degrees but in real life is it actually exactly 120 degrees.

Co32 Lewis Structure Carbonate Ion In 2021 Molecules Lewis Chemical Formula

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Boron

Po43 Molecular Geometry Shape And Bond Angles By Wayne Breslyn

Shapes Of Molecules Cie A Level Chemistry Revision Notes

A What Is The Value Of The Bond Angles In Clutch Prep
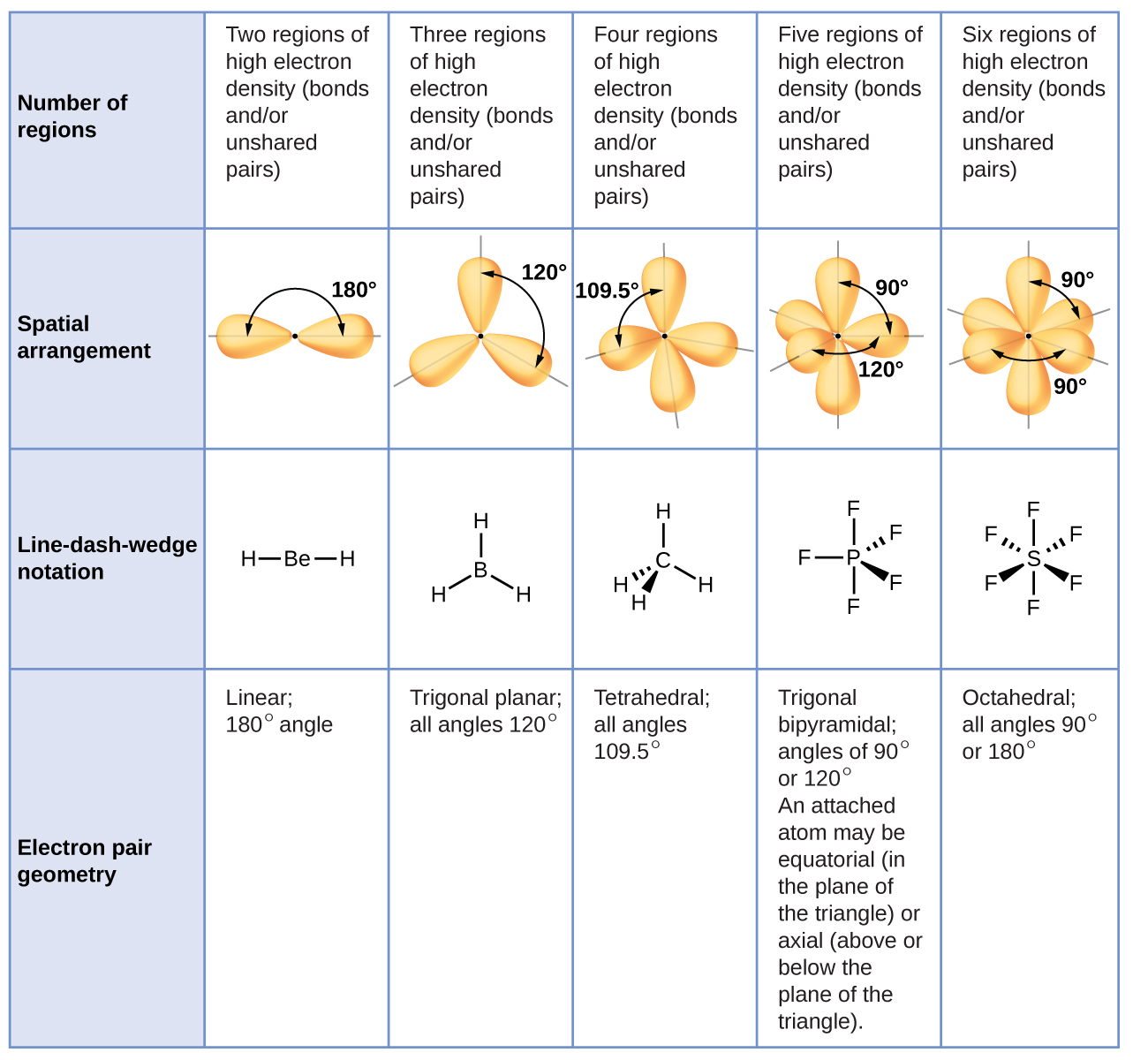
4 7 Molecular Structure And Polarity General Chemistry 1 2
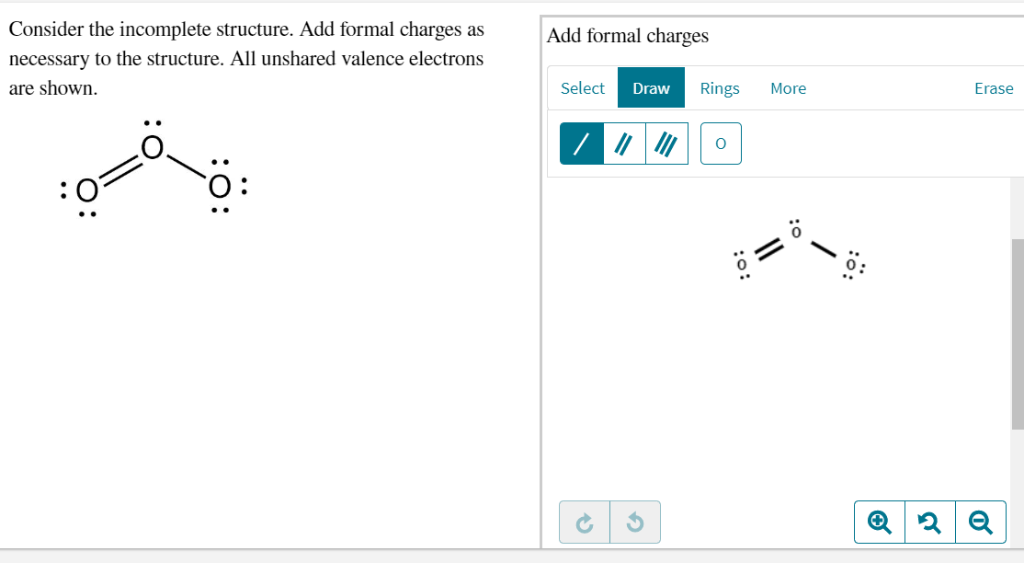
Solved Consider The Incomplete Structure Add Formal Charges Chegg Com

How Is Vsepr Theory Used To Predict Molecular Structure Ppt Video Online Download

A What Is The Value Of The Bond Angles In Clutch Prep
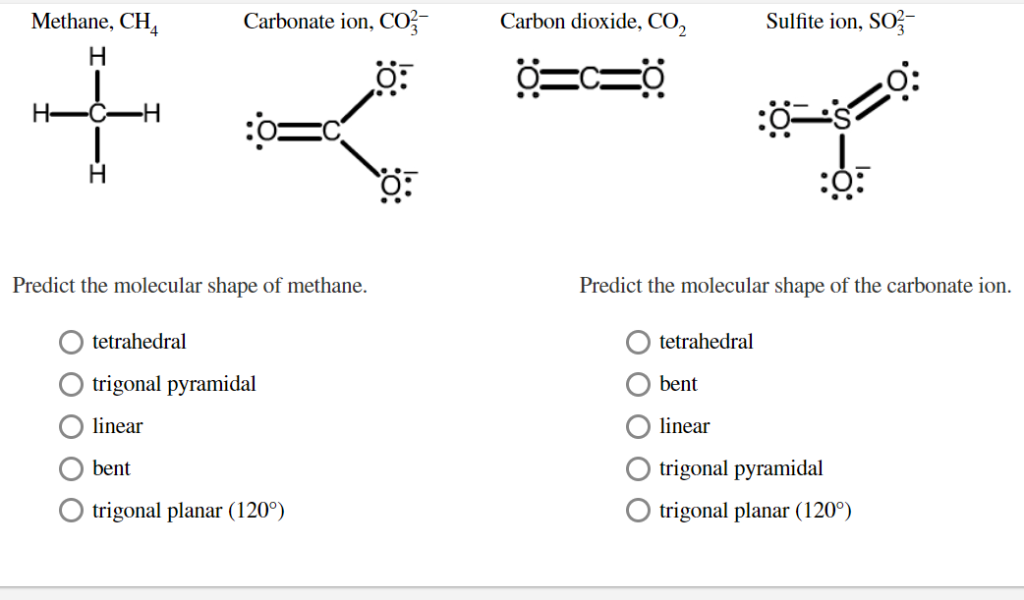
Solved Consider The Incomplete Structure Add Formal Charges Chegg Com

Xef4 Molecular Geometry Bond Angles Electron Geometry Xenon Tetrafluoride In 2021 Molecular Geometry Molecular Geometry

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion Youtube
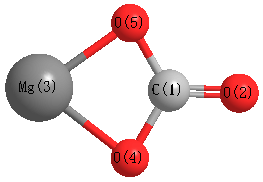
Cccbdb Compare Core Correlation Versus No Core Correlation On Bond Lengths
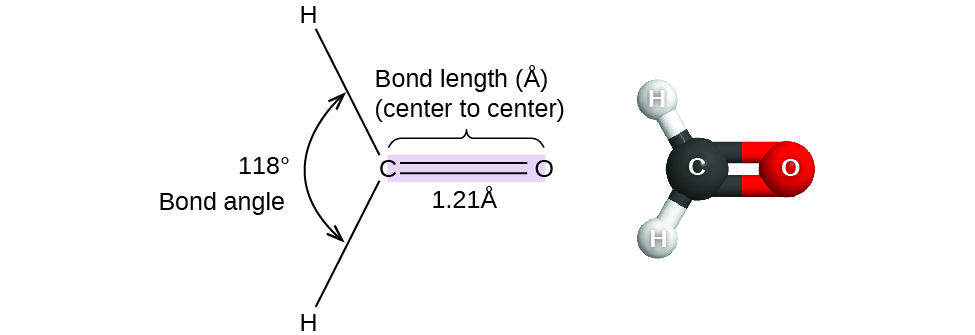
7 6 Molecular Structure And Polarity Chemistry Libretexts
Posting Komentar untuk "Carbonate Ion Bond Angle"