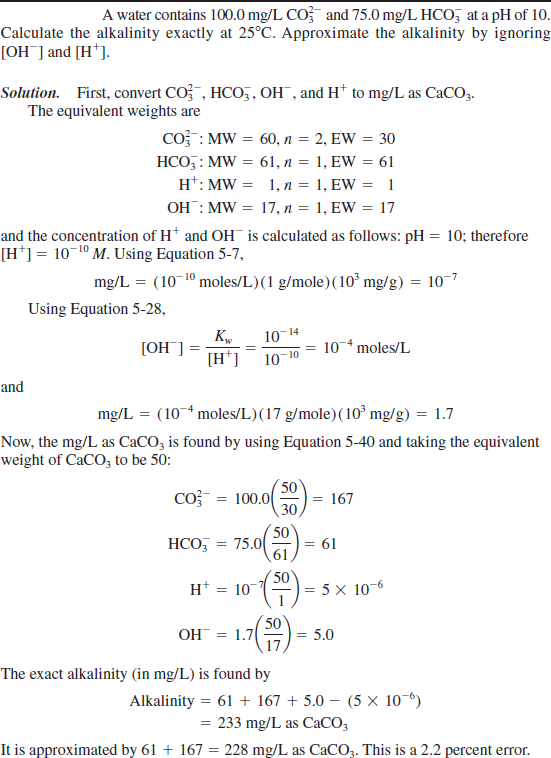
Carbonate Alkalinity Formula
Most people refer to alkalinity as the concentrations of carbonate CO 3 2- and bicarbonate HCO 3 ions which are the buffers that are typically present in the highest concentrations in natural waters. Nonetheless I have long noted that Ward Labs alkalinity bicarbonate and carbonate numbers do not jive.
Bicarbonate is the major form of alkalinity.
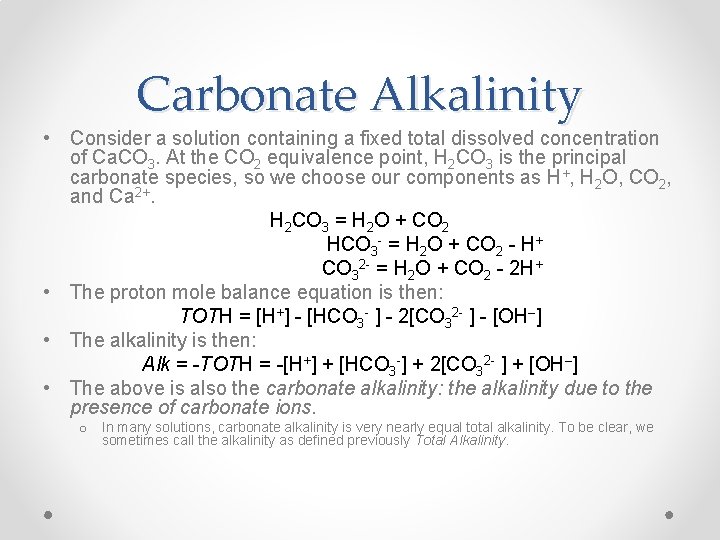
Carbonate alkalinity formula. The formula for the general case is given here which is used in standard hydrochemistry programs including aqion. It is an aggregate measure of the sum of all titratable bases in the sample. This is the endpoint for carbonate alkalinity and CO 2 acidity titrations.
All forms are white odourless water-soluble salts that yield moderately alkaline solutions in water. Carbonate Alkalinity as CO 3 2-mgL 06 Carbonate Alkalinity as CaCO 3 mgL Converting Bicarbonate Alkalinity from mgL as CaCO 3 to mgL as HCO 3-Consider the following reaction. Alkalinity is a measure of the acid-neutralizing capacity of water.
The unit of measurement for alkalinity is. Total alkalinity is determined by titration to a pH of 51 48 45 or 37 depending upon the amount of carbon dioxide present. CaCO 3 H 2O CO 2 Æ CaHCO 3 2 CaCO 3 has a molecular weight of 100 gmol The HCO 3-anion has a molecular weight of 61 gmol Therefore each mol of CaHCO 3.
Alkalinity divided by the atomic weight of calcium carbonate multiplied by 1000 multiplied by the twice the atomic weight of sodium bisulfate equals the amount per liter in grams of 100 sodium bisulfate to add per ppm increase desired. We then review different methods that have been used to solve the total alkalinitypH equation with a main focus on bio-geochemical models. Solving the equation yields AlkmgL as CaCO31968mgL.
Carbonate Alkalinity CA CA 2CO 3-2 HCO 3- Typically HCO 3-and CO 3-2 are present at 1000x conc of other proton acceptors Hence. We then present two variants of a new robust and universally convergent al-gorithm to solve the total alkalinitypH equation. Anyway heres the formula.
Carbonate CO 3 2- Alkalinity as CaCO 3 Bicarbonate HCO 3- Alkalinity as CaCO 3 p alk 0 0 0 t alk p alk ½ t alk 0 2p alk t al 2p alk p alk ½ t alk 0 2p alk 0 p alk ½ t alk 2p alk t alk 2t alk p alk 0 p alk t alk t alk 0 0 MANTECHs MT Series automates alkalinity and low alkalinity measurements following EPA ASTM ISO and Standard Methods. The equations above are restricted to carbonate systems without other weak acids or bases. Calcium carbonate CaCO3 equivalent Alkalinity test results are usually reported as mgL CaCO3 equivalents.
The shortcomings and limitations of these methods are made out and discussed. The total alkalinity includes all carbonate-bicarbonate alkalinity and hydroxide alkalinity. Yes you read that right.
1 x 2401112 0002398952 100091000 This amount multiplied by the conversion. To calculate alkalinity as calcium carbonate use this formula to calculate the alkalinity in concentration of calcium carbonate CaCO3. CA nearly equals TA Calculations Any two of the four CO 2 properties ΣCO 2 P CO2 pH and carbonate alkalinity can be used to determine the CO 2 system Traditionally pH and alkalinity were measured.
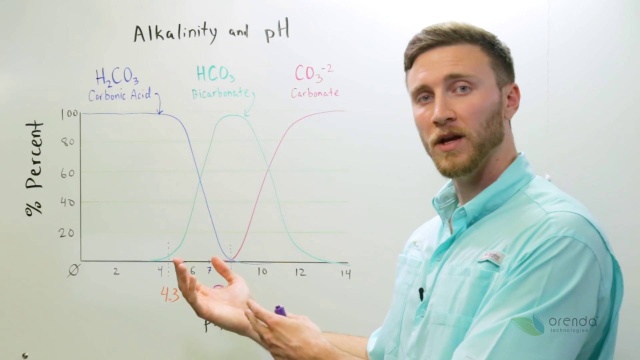
Apparently some chemists decided this topic was not confusing enough as it is. Alkalinity prevents sudden changes in the acidity level of water and hence is important for fish and. 2 At pH 83 the H 2CO 3 equals the CO 3 2-.
Sodium carbonate Na 2 CO 3 10H 2 O also known as Natrium Carbonate washing soda soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates. For example I found a Ward Labs report under Brew Science where the Alkalinity is listed as 199 the bicarbonate as 231 and the carbonate as 6. We dont want to get too nerdy.
Point and is the endpoint of the caustic alkalinity and total acidity titrations. Alkalinity d Bicarbonate and Hydroxide alkalinities cannot be present together e All hydroxide alkalinity is neutralized by pH 100 f all Carbonates are converted to bicarbonates by pH 83 Group Result of Hydroxide Carbonate Bicarbonate Titration Alkalinity Alkalinity Alkalinity A P 0 0 0 T initial pH 83 B P 05T 0 2P 0 C P T T 0 0. As we assumed all carbonate came from calcium carbonate we can write.
How do you calculate CaCO3 equivalent. Tion based upon carbonate-borate-alkalinity is presented. Cs ceCaCO3 ceH2CO3 HCO3- CO32- Where Cs here stands for the known concentration of the salt calcium carbonate.
In the alkalinity titration virtually all of the CO 3 2-has reacted thus the term carbonate alkalinity and half of. 15 10 15 ppm CaCO3. Section 5- Carbonate Chemistry 22 AlkalinityOHHCO 3 2CO 3 OHHCO 332CO 18 Therefore there is no alkalinity in a CO 2-water system unless other sources of base are added.
CaCO 3-CO 2-H 2 O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide. Alkalinity in most natural waters is due to the presence of carbonate CO 3 bicarbonate HCO 3- and hydroxyl OH- anions. Soda ash also known as sodium carbonate Na2CO3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate-bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash.
Titration with a strong acid e. Bicarbonate in particular is the strongest buffer largest Ka value and the effect of other buffers becomes insignificant in its presence. Note that this simple formula requires that you use the exact acid concentration and sample volume listed in this procedure.
Carbonate Alkalinity CaCO 3 mgL 100000 K 2 HCO 3-10 pH And if you really want to convert carbonate alkalinity from units of CaCO 3 to units of CO 3 2- this source shows you how to do so. ML of acid 10 ppmmL For example if 15 mL of acid were used in the titration then the total alkalinity would be. Alkalinity of natural water is mainly due to the presence of two forms of the carbonate ions denoted as HCO3- and CO32- that act as a buffer system.
M P H 2 CO 3 HCO 3- CO 3-2 DIC. G HCl Guy Munhoven Chemical Equilibria and pH Calculations Introduction Carbonate Chemistry pH Calculation Chemical Equilibria pH Scales Speciation Alkalinity State Variables of the Carbonate System H or pH and CO 2aq or pCO 2 are the only species participating in the carbonate equilibria.

Carbonate System Alkalinity Lecture 21 Toth Toth Is
Carbonate Alkalinity Vs Corrected Alkalinity
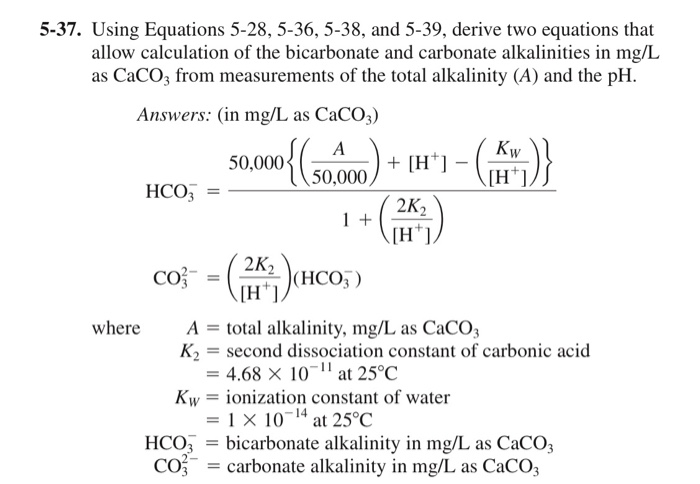
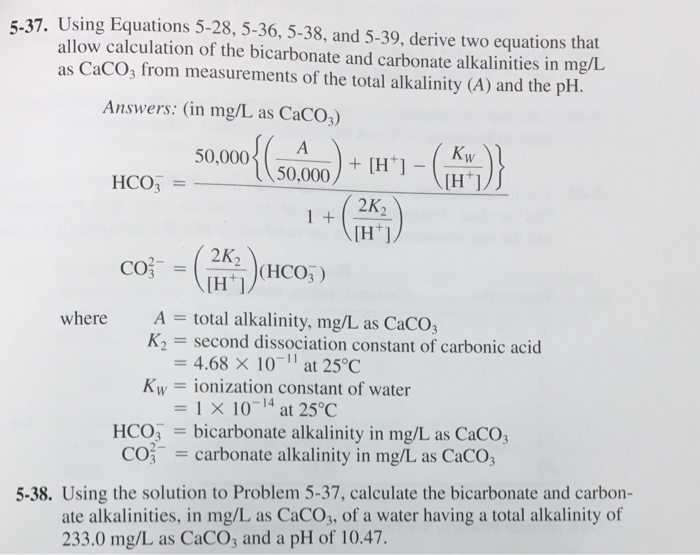
Using Equations 5 28 5 36 5 38 And 5 39 Derive Chegg Com
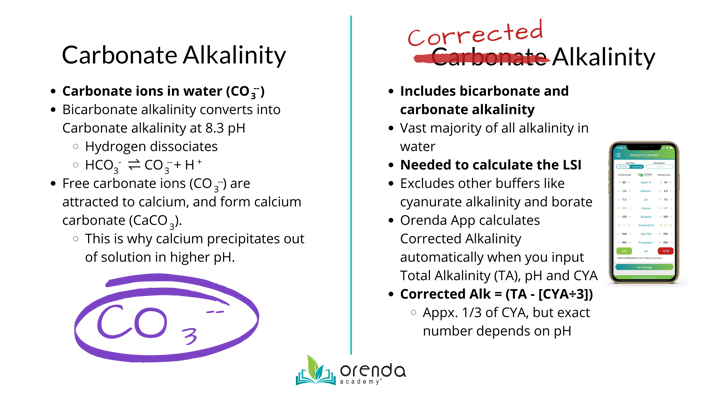
Carbonate Alkalinity Vs Corrected Alkalinity

Is There Corelation Between Ph Alkalinity And Bicarbonate In Water
Solved 5 37 Using Equations 5 28 5 36 5 38 And 5 39 Chegg Com

The Geochemistry Of Natural Waters The Carbonate System

Carbonate System Alkalinity Lecture 21 Toth Toth Is The Total Amount Of Component H Rather Than The Total Of The Species H O Every Species Containing Ppt Download

Ppt Carbonate System Alkalinity Powerpoint Presentation Free Download Id 1837976

Carbonate Alkalinity Vs Corrected Alkalinity


Posting Komentar untuk "Carbonate Alkalinity Formula"