Carbonate Net Charge
Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. Ionic Equations for Neutralisation.
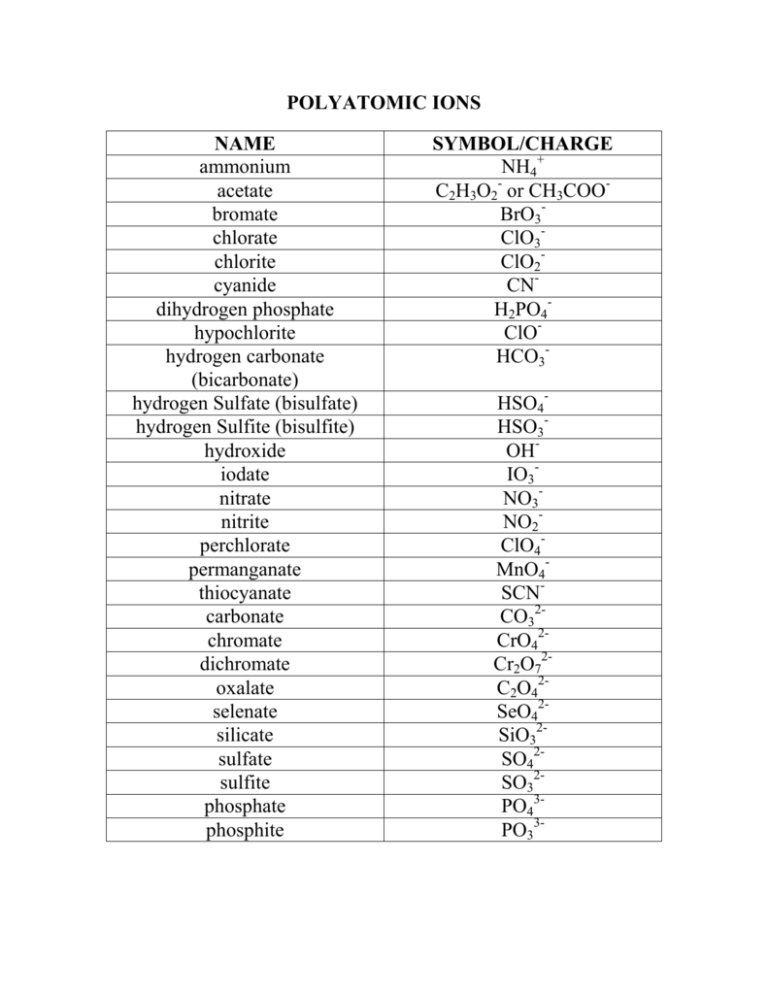
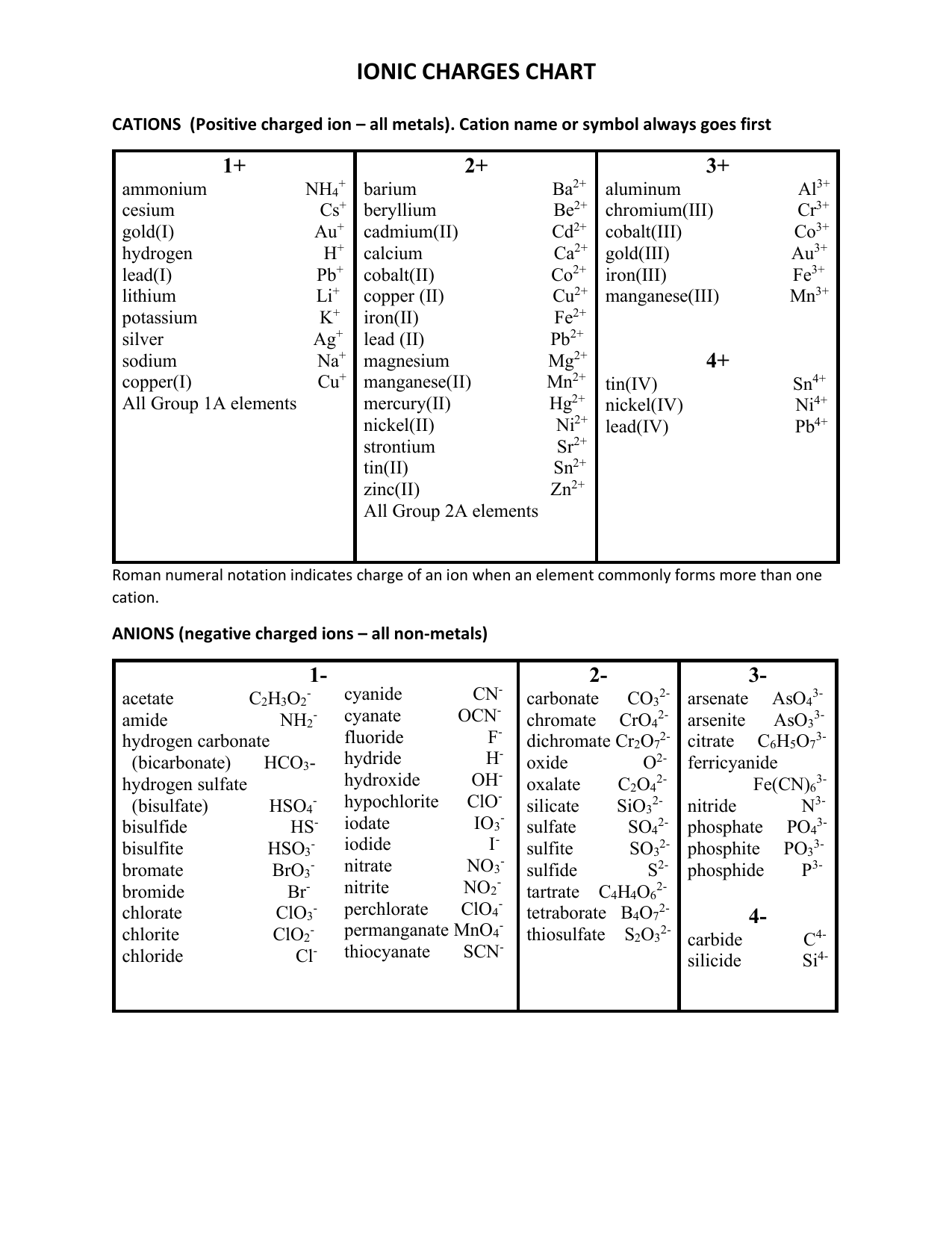
Polyatomic Ions Name Symbol Charge Ammonium Nh4
Carbonate Ion is a polyatomic ion with formula of CO3 2-.
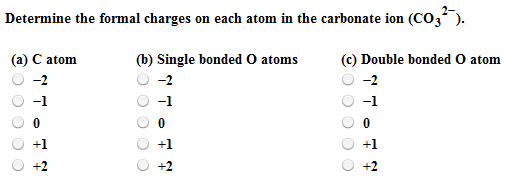
Carbonate net charge. Sodium carbonate Na 2 CO 3 Potassium carbonate K 2 CO 3. In this video we will describe the equation Na2CO3 H2O and write what happens when Na2CO3 is dissolved in waterWhen Na2CO3 is dissolved in H2O water it. Calculating the net charge.
The net charge is the charge that there is more of in an object. We can simply find the charge by summation of charges of each atom molecule used to form the compound. Carbonate CO 3 2- has a negative charge.
A carbonate salt forms when a positively charged ion M M 2 or M 3 associates with the negatively charged oxygen atoms of the ion by forming electrostatic attractions with them forming an ionic compound. Under some circumstances protons and electrons can be converted to other particles in certain nuclear reactions but in doing so the net charge for the reactions is zero. Ni2 nickelII OH-hydroxide.
The charge distribution of carbonate ions in calcite and in aragonite have been calculated on the principle that the crystal experiences no net expansive or compressive force and no ion within it experiences a net force or couple. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. 2 M CO 2 3 M 2 CO 3 M 2 CO 2 3 MCO 3 2 M 3 3 CO 2 3 M 2 CO 3 3.
Each sodium cation holds a charge of 1 whereas the polyatomic carbonate anion holds a. An atom of an element is most stable when its outer electron shell is completely filled or half-filled. The charges q in e for calcite are q C 095026 q O 098009 and for aragonite q C 089 q O 1 094 and q O 2 098 errors.
2 leadII CO 3. A carbonate ion is made of one carbon atom and three oxygen atomsThe carbon is the cation and has a charge of 4 while the oxygen becomes the anion with a charge of -2. In the carbonate ion the carbon atom is bonded with a double bond to an oxygen atom and with single bonds to two oxygen atoms.
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Furthermore What is the charge on a carbonate ion The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. It is a conjugate base of a hydrogencarbonate.
The consensus that negatively charged functional groups are predominant over positively charged groups in microbial cell walls is widely accepted Corpe 1970. The most common charges are based on maximum stability for the atom. 2 ironII NO 2-nitrite Cu.
It is quite simple the charge on Ca Ion is 2 and the charge on CO3 is 2- so net sum of charge on the CaCO3 compound is 2-20 it is neutral. Charge Polyatomic Ions To. This means that we will split them apart in the net ionic equation.
For example ironII has a 2 charge. 2 copperII NO 3-nitrate Cu copperI SO 3 2-sulfite Cr. 3 chromiumIII SO 4.
The charges are balanced whereby the total charge on the left is equal to the total charge on the right. 1 Charge 2 Charge Ammonium NH 4 Chromyl CrO 2 2 Nitronium NO 2 MercuryI Hg 2 2 Hydronium H 3 O Uranyl UO 2 2 Pervanadyl VO 2 Vanadyl VO2. Pb4 leadIV PO 4 3-phosphate Pb.
The carbonate ion CO3 2- has an overall charge of -2. It can be noted that each molecule of sodium carbonate contains 2 sodium atoms 3 oxygen atoms and one carbon atom. Carbonate is made of 1 atom of carbon and 3 atoms of oxygen and has an electric charge of 2.
Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-bicarbonate HCO 3-hydride H-. This negative charge means that a single ion of carbonate has 2. Both Na2CO3 and AgNO3 are considered strong electrolytes and will dissociate completely.
Therefore the net ionic equation will show the actual chemical change without the spectator ions. However other charges are possible. 3 ironIII NH 4 ammonium Fe.
IronIII a 3 charge. While it is a good model to think of conservation as an inability to increase or decrease the total number of protons and electrons it technically isnt 100 accurate. Hg2 mercuryII ClO 3-chlorate.
Because of these opposite charges these ions naturally pair with one another similar to two magnets. C 2H 3O 2-acetate. Subsequently the presence of both positively and negatively charged functional groups implies a changing net charge depending on matrix pH Van Der Wal et al 1997.
The structure of sodium carbonate molecules is illustrated below. Can AgNO3 react with Na2CO3. So the formula for carbonate is CO₃ 2- Formula for Carbonate In carbonate ionthe oxidation number of carbon is 4 and the oxidation number of O is -2The net charge of carbonate is -2.
The result is a white precipitate. The charge on an atom is related to its valence electrons or oxidation state. Silver Nitrate with Sodium Carbonate Here sodium carbonate Na2CO3 is added to silver nitrate AgNO3.
Because of these opposite charges these ions naturally pair with one another similar to two magnets. For example if an object has 5 electrons negative charges and 4 protons positive charges then the net charge is negative.
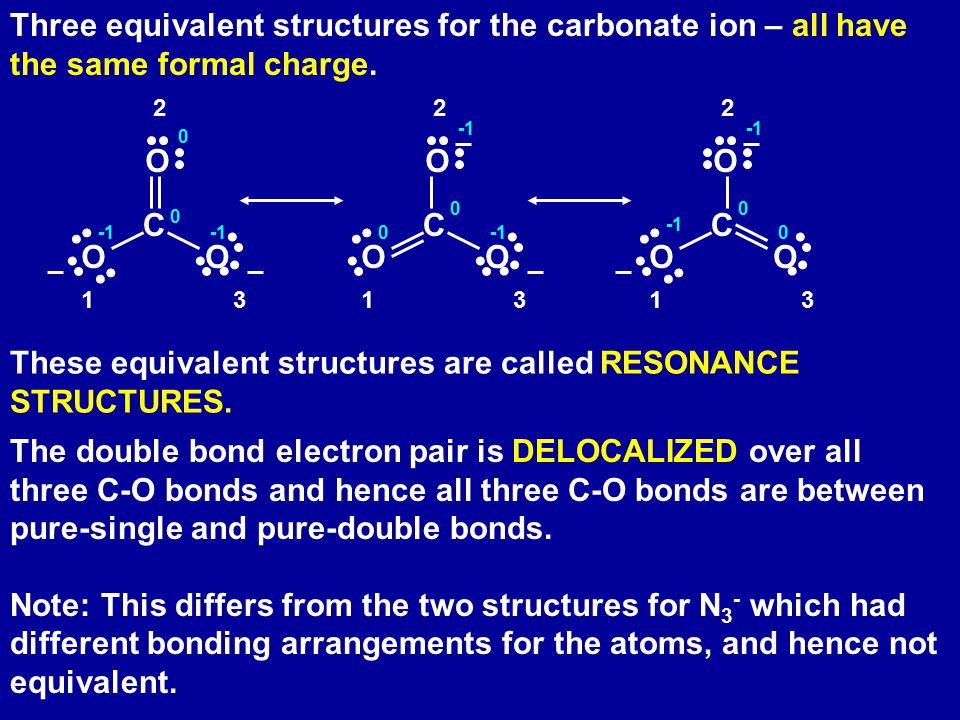
Example Co3 Carbonate Ion Ppt Download
What Is The Charge Of Co3 Science Trends
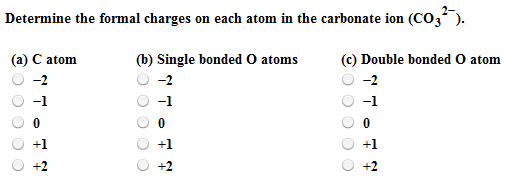
Solved Determine The Formal Charges On Each Atom In The Chegg Com

How To Write The Net Ionic Equation For Cacl2 Na2co3 Caco3 Nacl Youtube
The Carbon Cycle Carbon Dioxide And Carbonate System

K2co3 Is An Ionic Compound Cation Is Potassium Ion K Ppt Download

Is Co32 Polar Or Nonpolar Carbonate Ion Polarity Geometry Of Molecules
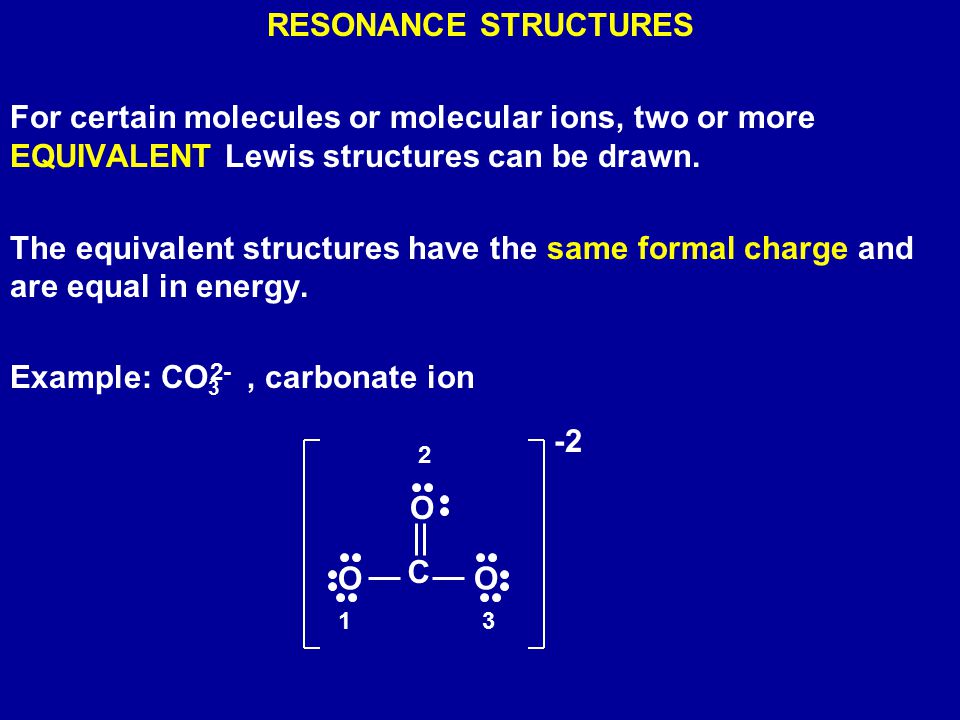
Example Co3 Carbonate Ion Ppt Download

Topic Polyatomic Ions Ppt Download
How To Calculate The Formal Charges For Co3 2 Carbonate Ion Youtube

How To Write The Formula For Potassium Carbonate K2co3 Youtube
Posting Komentar untuk "Carbonate Net Charge"