Zinc Carbonate + Hydrochloric Acid
Magnesium hydroxide hydrochloric acid magnesium chloride water. A Zinc hydroxide b Sodium zincate c Zinc hydrogenate d No reaction takes place.
Calcium Carbonate A Guide For Gcse Students Knockhardy
ZnCO3 2HCl ZnCl2 CO2 H2O.

Zinc carbonate + hydrochloric acid. Zn HCl ZnCl₂ H₂. Hydrochloric acid copper carbonate copper chloride water carbon dioxide. When a colorless solution of lead II nitrate is added to a colorless solution of potassium iodide a yellow solid called a precipitate is instantly produced.
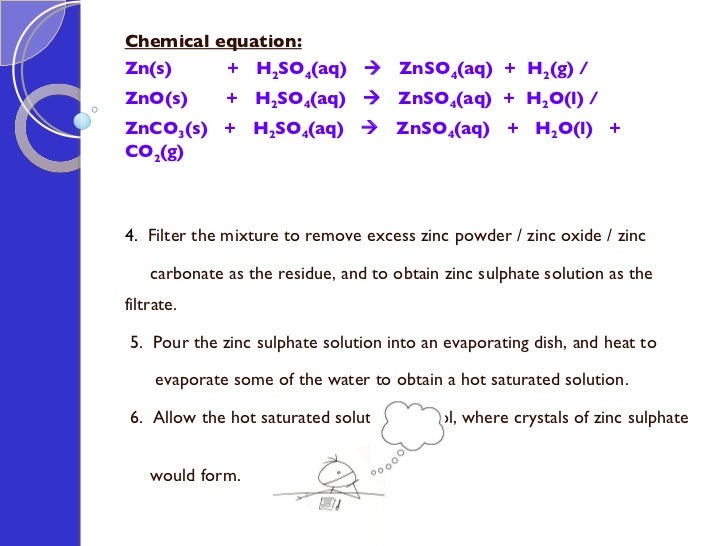
Zn s H 2 SO 4 aq ZnSO 4 aq H 2 g Acid reacting with. Get an answer for Balanced equation of zinc carbonate nitric acid zinc nitrate carbon dioxide water and find homework help for other Science questions at eNotes. The chemical equation for the reaction can be given as rmZnCrmO_3 2rmHCl to rmZnCrml_2 rmCrmO_2 rmH_2rmO.
Acid Metals Salt Hydrogen Gas. Answer b Zinc chloride Register here for free tests. 2HClaq CuCO 3 s CuCl 2.
A student performed an experiment using zinc granules and sodium carbonate with sodium hydroxide and hydrochloric acid under different conditions as shown here. See the answer See the answer See the answer done loading. Show transcribed image text.
Reaction of zinc and hydrochloric acid. Lets examine the example of the interaction between zinc and hydrochloric acid. But treating hydrogen sulfide gas to calcium chloride and zinc chloride can be used to identify solutions.
Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. He would observe that no gas is evolved in the setup. CopperII carbonate sulfuric acid copperII sulfate water carbon dioxide.
A Zinc sulphate b Zinc chloride c Zinc carbonate d Zinc hydroxide. Which of the following compound is formed when zinc reacts with hydrochloric acid a Zinc sulphale b Zinc chloride c Zinc carbonate d Zinc hydroxide. Chemical Properties of Zinc Carbonate ZnCO 3.
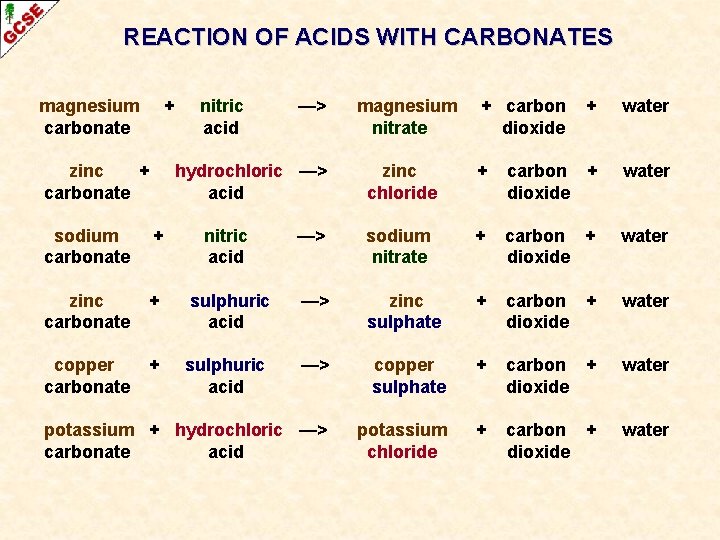
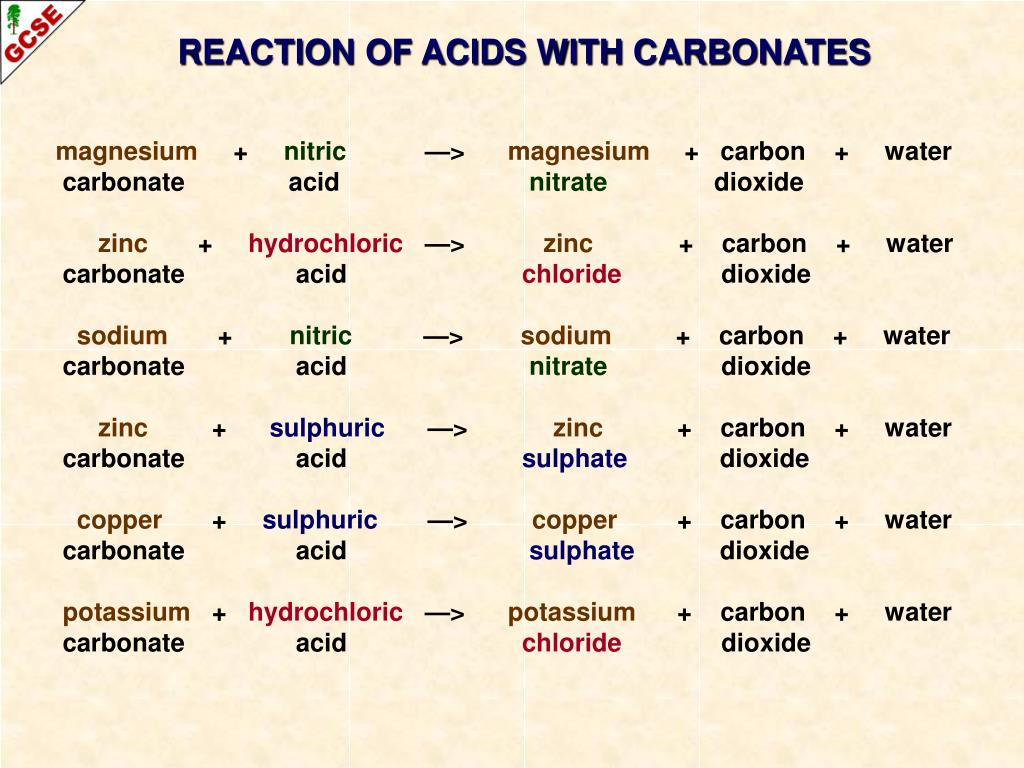
Acid carbonate salt water carbon dioxide. A metal carbonate X on reacting with acid gives a gas which when passed through a solution Y gives the carbonate back. Magnesium oxide nitric acid magnesium nitrate water.
What are the gases liberated when dilute hydrochloric acid reacts with 1 zinc 2 zinc carbonate. The chemical reactions is as below. Determine the net ionic equation for this reaction.
Which of the following compound is formed when zinc reacts with hydrochloric acid. Neutralization reaction is defined as the chemical reaction. Zinc also reacts with HCl releasing small bubbles of hydrogen and forming zinc chloride ZnCl₂.
The reaction between zinc and hydrochloric acid is Z n 2 H C l Z n C l 2 H 2 This reaction is known as single replacement reaction where zinc metal displaces the hydrogen to form hydrogen gas and zinc chloride a salt. Calcium sulfide is soluble in water. Determine the molecular equation for the reaction of zinc carbonate with hydrochloric acid.
Instead of dilute sulphuric acid if we use dilute hydrochloric acid ZINC Chloride is formed Zn 2HCl ZnCl 2 H 2. Zinc sulfuric acid zinc sulfate hydrogen. Zinc carbonate reacts with acids like hydrochloric acid forms zinc chloride and release carbon dioxide.
R e a s o n. What is formed when zinc carbonate reacts with concentrated hydrochloric acid. Zinc hydrochloric acid zinc chloride hydrogen.
When Zinc Zn reacts with dilute Sulphuric Acid H 2 SO 4 it produces a salt called Zinc Sulphate ZnSO 4 and Hydrogen Gas. This problem has been solved. Zinc carbonate reacts with hydrochloric acid to form zinc chloride and water along with the liberation of carbon dioxide gas.
Calcium carbonate and zinc carbonate reacts with dilute hydrochloric acid and emit carbon dioxide gas form colorless solutions. Zinc reacting with hydrochloric acid produces bubbles of hydrogen gas. Which of the following compound is formed when zinc reacts with sodium hydroxide.
Download our all new app for latest notes and useful assignments. On the other hand a gas G that is obtained at the anode during electrolysis of brine is passed on dry. In this video well balance the equation HCl ZnCO3 ZnCl2 CO2 H2O and provide the correct coefficients for each compoundTo balance HCl ZnCO3 ZnCl.
Click here to register. CaCl 2 and ZnCl 2 are colorless solutions.

How To Balance Hcl Znco3 Zncl2 Co2 H2o Hydrochloric Acid Zinc Carbonate Youtube
Ppt Calcium Carbonate A Guide For Gcse Students Powerpoint Presentation Id 1487993

Ppt Do Now Powerpoint Presentation Free Download Id 1560113
Solved Solid Zinc Ii Carbonate Reacts With Aqueous Chegg Com

How To Balance H2so4 Zn Znso4 H2 Sulfuric Acid Zinc Youtube

How To Write The Net Ionic Equation For Hcl Znco3 Zncl2 Co2 H2o Youtube
What Happens When Zinc Reacts With Sulphuric Acid Quora

Zinc Carbonate Structure Properties And Uses Of Znco3

Seminar Form 4 Revision On Intro To Chemistry Structure Of Atoms F
How To Write Ammonium Carbonate Chemical Formula
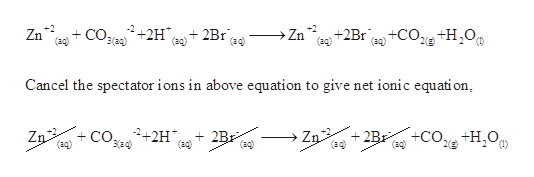
Answered Write A Net Ionic Equation For The Bartleby
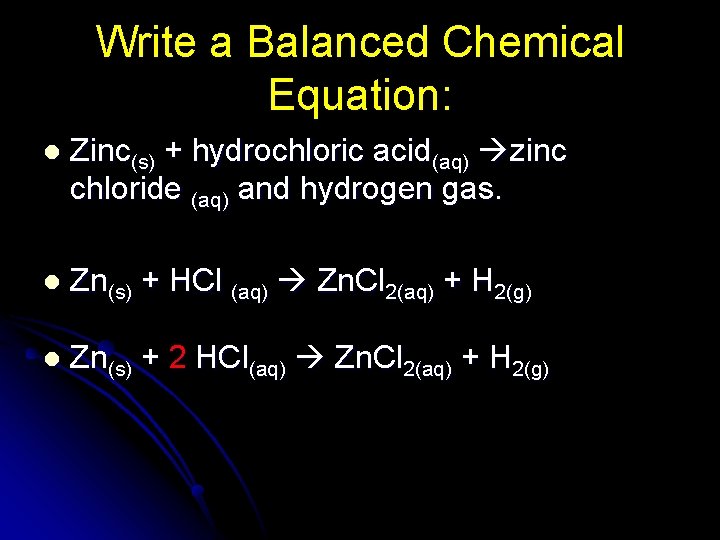
Chemical Equations And Reactions Chemical Reactions L A

Ppt Metal Reactions And Reactivity Powerpoint Presentation Free Download Id 3099937

Midterm Reaction Review Ppt Video Online Download

Posting Komentar untuk "Zinc Carbonate + Hydrochloric Acid"