Aluminium Carbonate Ionic Formula
B What is the molarity of a solution of aluminum chloride if 300 mathrmmL is required to react with 355 mathrmmL of 0137 mathrmM sodium carbonate. HCl ------ H 1 Cl -1.

03 Writing Ionic Formula Aluminium Carbonate Youtube
The criss-cross method of involving valencies is only useful for the formation of ionic compounds and not molecular or covalent compounds.

Aluminium carbonate ionic formula. Aluminium generally has a charge of 3. Writing ionic formula polyatomic Aluminium Carbonate About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features 2021 Google LLC. Why does Aluminium carbonate not exist.
2Al 3CuCl 2 2AlCl 3 3Cu. The ionic equations for the reaction are. Aluminum carbonate is Al2CO33.
The compound has a molar mass of 234gmol so 234x011627144 grams. In this video well balance the equation AlNO33 K2CO3 Al2CO33 KNO3 and provide the correct coefficients for each compoundTo balance AlNO33 K2C. Laboratory Chemical Safety Summary LCSS Datasheet.
Some common ways are by reacting the aluminium metal with hydrogen chloride or by conducting a single displacement reaction between copper chloride and aluminium metal. A Write the net ionic equation for this reaction. The electron configuration of Aluminium is 1s22s22p63s23p1.
CID 5359268 Aluminum CID 767 Carbonic acid Dates. The formula for aluminum fluoride is. The reaction between metallic aluminum and hydrochloric acid is what is known as an oxidation-reduction reaction.
Well if I have done my sum rights there are. It is a double displacement reaction in which aluminum carbonate and hydrochloric acid exchange their components. 2Al 3Cl 2 2AlCl 3.
CID 767 Carbonic acid Component Compounds. Choose from 500 different sets of ionic formula flashcards on Quizlet. Cations of hydrochloric acid take these electrons and are.
Carbonic acid aluminium salt. Learn ionic formula with free interactive flashcards. That means that there is 1 Aluminum and 3 fluorde ions in 1 molecule.
It can be manufactured with the high pressure of carbon dioxide and temperature close to 0C. In this video well balance the equation Al2CO33 HNO3 AlNO33 H2O CO2 and provide the correct coefficients for each compoundTo balance Al2CO33. Al 2 CO 3 3 ------ 2Al 3 3CO 3-2.
3O 3 2 6. This saves time during exams and helps in the general understanding of chemistry. Aluminium Carbonate Formula Aluminium carbonate is a carbonate of an aluminium salt with a chemical formula Al 2 CO 3 3 that doesnt exist in normal conditions.
Aluminum acts as the reducing agent giving up electrons. 2Al 6HCl 2AlCl 3 H 2. Carbonic acid aluminum salt.
Aluminum carbonate basic USAN Basaljel TN 1339-92-0. The Carbonate ion has a charge of -2. AlHCO33To write the formula for Aluminum bicarbonat.
Carbonic acid aluminium salt. Log in Sign up. 14 atom in the one formula unit of aluminium carbonate.
The 3p electron and the 2 3s electrons are outer valance electrons and are lost to form the 3 charge. If you have 70x1022 particles of it then thats 70x1022602x1023 0116 moles. C 3 H 3 AlO 9.
The Answer is A. 4 6 2. Aluminum carbonate basic USAN EINECS 238-440-2.
Browse 500 sets of ionic formula flashcards Advanced. Aluminum ions react with carbonate ions to form an insoluble compound aluminum carbonate. The reactions for the same are given below.
It helps to become familiar with the formulae of the common compounds of chemistry. In this video well write the correct formula for Aluminum bicarbonate Aluminum hydrogen carbonate. Al⁰ - 3e Al³.
Formula unit is the empirical formula of an ionic or a. The 3 does not effect the Aluminum at all. On the other hand it is a crystalline solid that has no color or a white powder that has a strong odor of ammonia and has a very Sharp ammoniacal taste.
1C 1 4. The reason why aluminium carbonate cannot exist is because it is simply too unstable and would rather be broken down instead. Rightarrow Therefore two aluminum cations react with three carbonate ions to give aluminum carbonate that can be represented by the formula Al_2CO_3.
The chemical or the molecular formula for the Ammonium Carbonate is termed as NH42CO3. The carbonic acid which is produced during this reaction is unstable so it decomposes into carbon dioxide and water. In this video well write the correct formula for Aluminum carbonate Al2CO33To write the formula for Aluminum carbonate well use the Periodic Table a C.
The formula unit for aluminum carbonate is Al2CO33 this is because 3 carbonate ions exist for every two aluminum ions in the ionic compound. Chemical formula are written to show the relative numbers of atoms or ions in the simplest formula unit of the compound. Chemical compounds have a net charge of zero.

How To Write The Net Ionic Equation For Nh4 2co3 Bacl2 Nh4cl Baco3 Youtube
Ternary Ionic Compounds Naming And Formulas Ck 12 Foundation

How To Write The Formula For Aluminum Carbonate Youtube
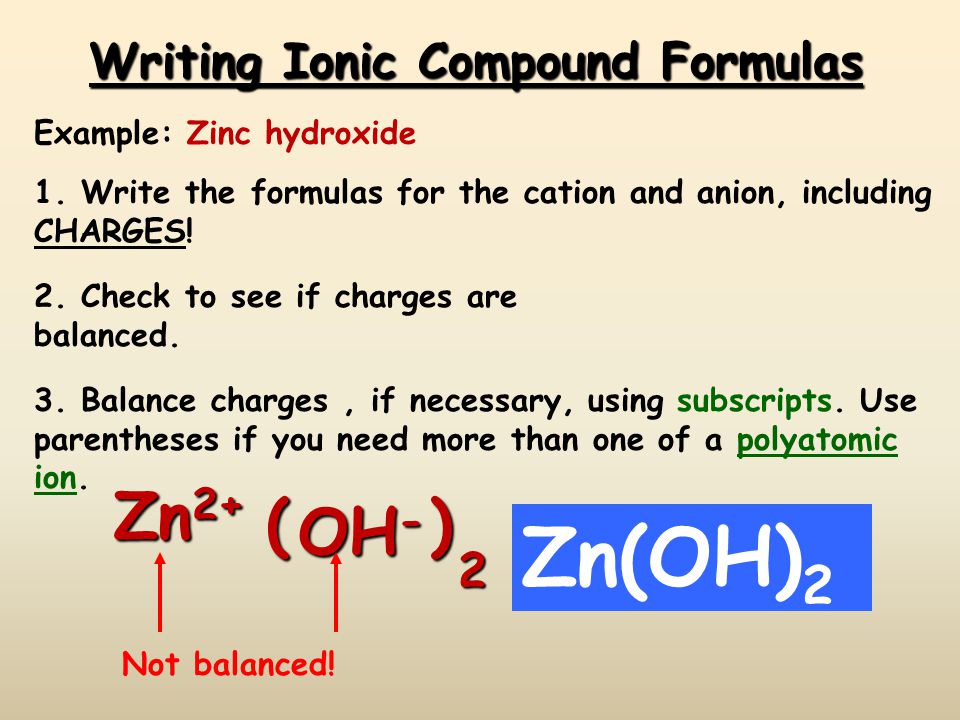
Ionic Compound Formulas Ppt Video Online Download
Chemistry Writing Ionic Formulae

Naming Ionic Compounds Acirc Euro Ldquo Answer Key Ionic Compounds Acirc Euro Ldquo Answer Key Give

Oneclass 1 Write A Net Ionic Equation For The Reaction That Occurs When Potassium Carbonate Aq An

Ionic Compound Formulas Ppt Video Online Download

Chapter 9 Chemical Names And Formulas Ppt Video Online Download
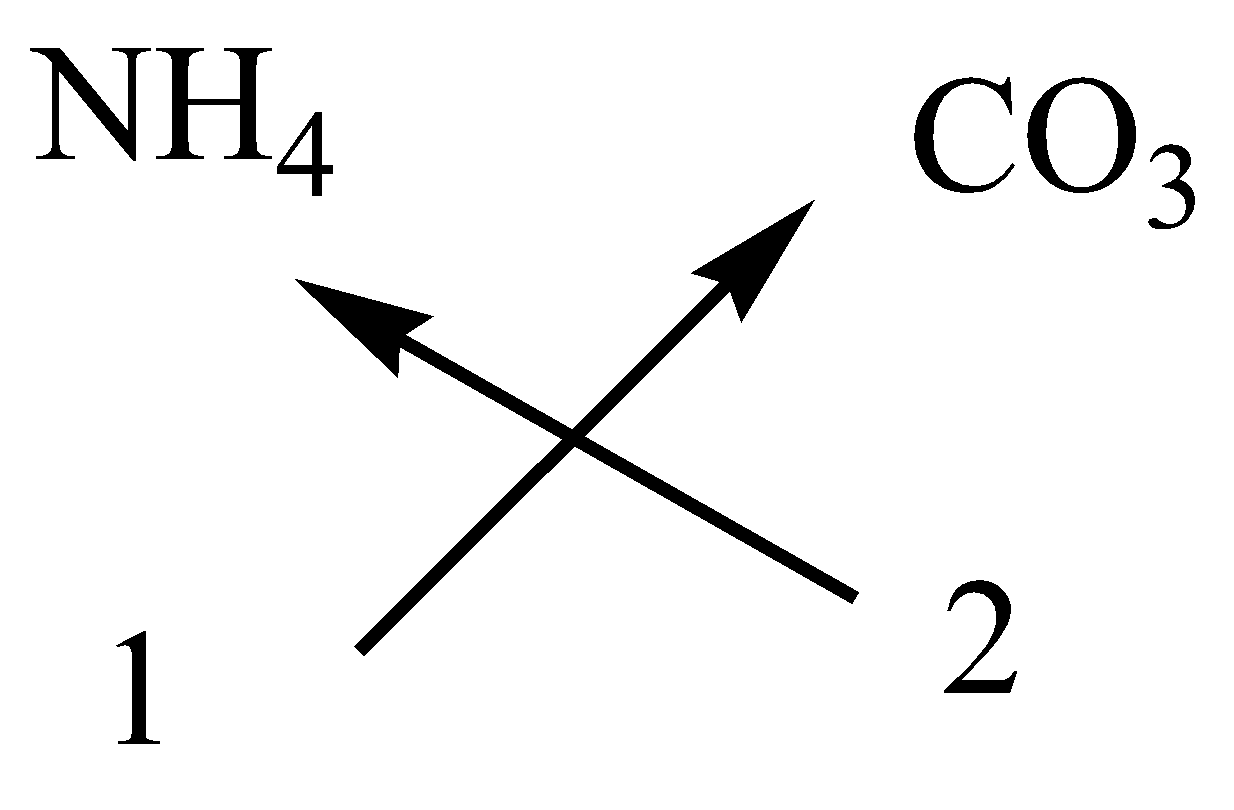
Write The Formula Of The Following Compounds By Criss Class 11 Chemistry Cbse

Write The Chemical Formula Of Ammonium Chloride Explain Why An Aueous Solution Of Ammonium Chloirde Youtube
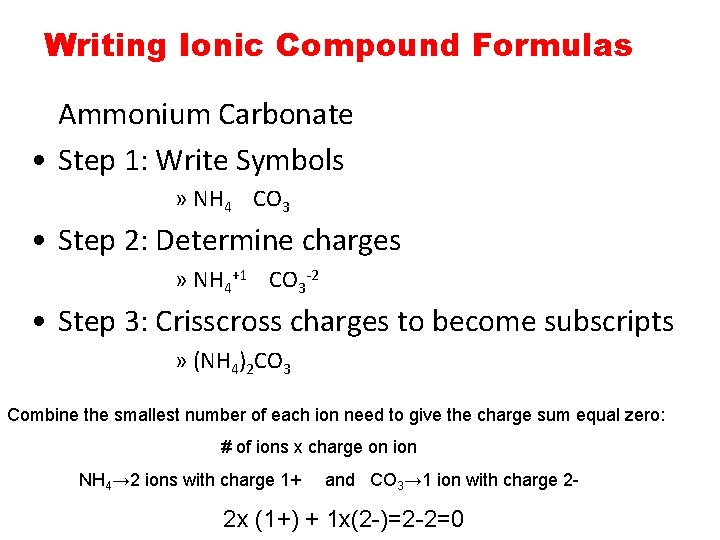
Chemical Bonding The Ionicbond Model Chemical Bonding The

Ionic Compound Formulas Ppt Video Online Download

Ionic Compound Formulas Ppt Video Online Download
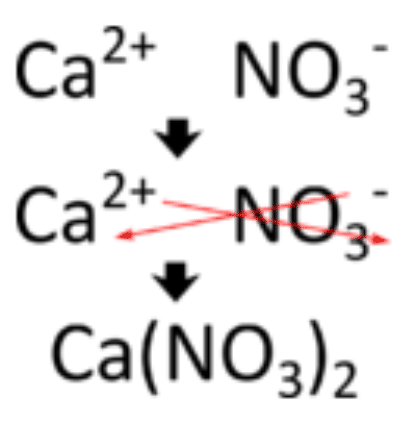
Posting Komentar untuk "Aluminium Carbonate Ionic Formula"