Carbonate Ion Lewis Structure Formal Charge
It consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D3h molecular symmetry. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.

Download 35 Possible Resonance Structures For Co32
It has a molecular mass of 6001 gmol and carries a total formal charge of 2.

Carbonate ion lewis structure formal charge. Add that all up. 7 7 0All atoms in BrCl 3 have a formal charge of zero and the sum of the formal charges totals zero as it. Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure.
Lets consider the Lewis structure of the carbonate ion CO32. The other two structures contribute less to the bonding structure 25 each. Discuss the shape of the following molecules using VSEPR model.
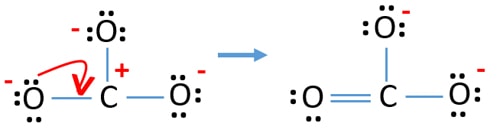
We can convert each lone pair to a bonding electron pair which gives each atom an octet of electrons and a formal charge of 0 by making three CC double bonds. Metal carbonate compounds are common in the world. Resonance Structures For Co2 Carbon Dioxide Youtube.
The formal charge of carbon atom in carbonate ion is-. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. 7 7 0Cl.
We will have to know that formal charge of an atom is not equal to real charge of an ion or a molecule. The central atom Be has only two bond. Assign A Formal Charge To Each Atom In The Students Lewis Structure.
With H CO 3- Carbon C is the least electronegative and goes in the center of the structure. Were still using only 24 valence electrons. BeCl 2 BCl 3 SiCl 4 AsF 5 H 2 S PH 3.
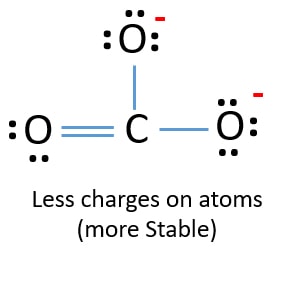
A structure in which the formal charges are as close to zero as possible is preferred. 4 plus 18 plus 2. But which of the three.
The Lewis structure for the carbonate ion is attached as shown. So -1 plus -1 that does match up with what we have for the carbonate ion here. A Student Proposes The Following Lewis Structure For The Carbonate CO2_3 Ion.
First we calculate the number of bonds needed. Each of the singly bonded oxygen atoms bears a formal charge of 1 and all other atoms are neutral. Lewis dot structure Cl.
Lets do the CO3 2- Lewis structure. Lewis structure of ion is Formal Charge on single bonded O atom. The Carbonate CO_32 Ion.
Carbonate ion has a -2 charge. This gives the formal chargeBr. Using the formula the formal charge for each element of the compound was determined as shown.
For the H CO 3-Lewis structure Carbonic Acid make sure you put the Hydrogen atom on the outside of an oxygen atoms. The carbonate ion is the simplest oxocarbon anionIt consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D 3h molecular symmetryIt has a molecular mass of 6001 gmol and carries a total formal charge of 2. Carbon is the least electronegative put that at the center.
The formula for the formal charge is also given. Calculate the FormalCharge on atoms in carbonate ion. Steps of drawing lewis structure of CO 3 2-Following steps are required to draw the CO 3 2-lewis structure and they are explained in detail in this.
Introduce an hydrogen ion carbonate becomes bicarbonate and the Lewis structure is OC-O-OH. There are three σ bonds and π bond around carbon atom in the Lewis structure of CO 3 2-ion. Carbonate ion a moderately strong base undergoes considerable hydrolysis in aqueous solution.
The carbonate dianion may be represented as OC-O-_2 with a formal negative charge on 2 oxygen atoms. Carbon has 4 valence electrons. In order to calculate the formal charges for CO3 2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e.
The lone pairs are the electron dots around the oxygen elements. You should put the H CO 3-Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. There are 24 valence electrons involved in the bonding.
Formal charge is only a theoretical charge over an individual atom of an ion a molecule. Co2 Lewis Structure Easy Hard Science. Because of that this is the best Lewis structure for CO3 2-.
CO 3 2-Lewis structure. Thus CO 2 has non-equivalent resonance structures. Is CO3 2 an acid or base.
So the formal charges do make sense here because the black Oxygens here and here both have a formal charge of negative 1 and the Carbon and the other Oxygen are 0. Three carbon atoms now have an octet configuration and a formal charge of 1 while three carbon atoms have only 6 electrons and a formal charge of 1. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure.
Oxygen has six we have 3 Oxygens and this negative 2 means we have an extra two valence electrons. Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. In a Lewis structure formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom.
As an CaCO 3 can be given. Formal Charge Lewis Structure Resonance Chemistry Carbon Dioxide Png 735x540px Formal Charge Area Atom Black And. The correct Lewis structure for this ion has one carbonoxygen double bond and two carbonoxygen single bonds.
The formal charge on carbon atom in carbonate ion is. The carbonate ion is the simplest oxocarbon anion. The formal charge lies on the oxygen atom.
Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube. Computational chemistry predicts that the left structure which minimizes formal charge since all atoms have a formal charge of zero contributes the greatest to the bonding in CO 2 50.

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube
What Is The Lewis Structure Of Co3 2 Quora
Lewis Structure For Co32 Carbonate Ion

Download Lewis Dot Structure Of Co3 Mp3 Mp4 Unlimited Loger Mp3

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Lewis Structure For Co32 Carbonate Ion

Co32 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Formal Charges And Resonance Chemistry Atoms First
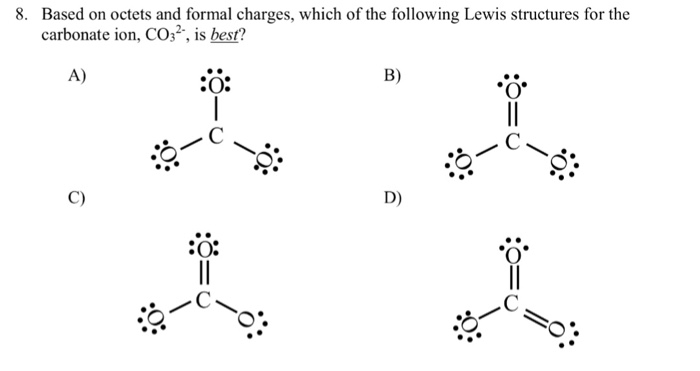
Solved Based On Octets And Formal Charges Which Of The Chegg Com

Draw The Structure Of Co32 Include All Lone Pairs Of Electrons And Formal Charges Draw The Ion By Placing Atoms On The Grid And Connecting Them With Bonds Include All Lone Pairs

Draw The Structure Of Co32 Include All Lone Pairs Of Electrons And Formal Charges Brainly Com
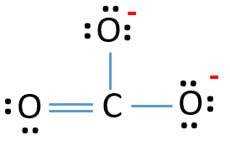
Lewis Structure For Co32 Carbonate Ion
Posting Komentar untuk "Carbonate Ion Lewis Structure Formal Charge"