Carbonate Ion Resonance Structures
Drawing correct lewis structure is important to draw resonance structures of CO 3 2-correctly. They only differ in the position of bonding and lone pair of electrons.

Co32 Molecular Geometry Shape And Bond Angles Carbonate Ion In 2021 Molecular Geometry Molecular Geometry
A resonance structure means that there are more than one way to draw the ion.
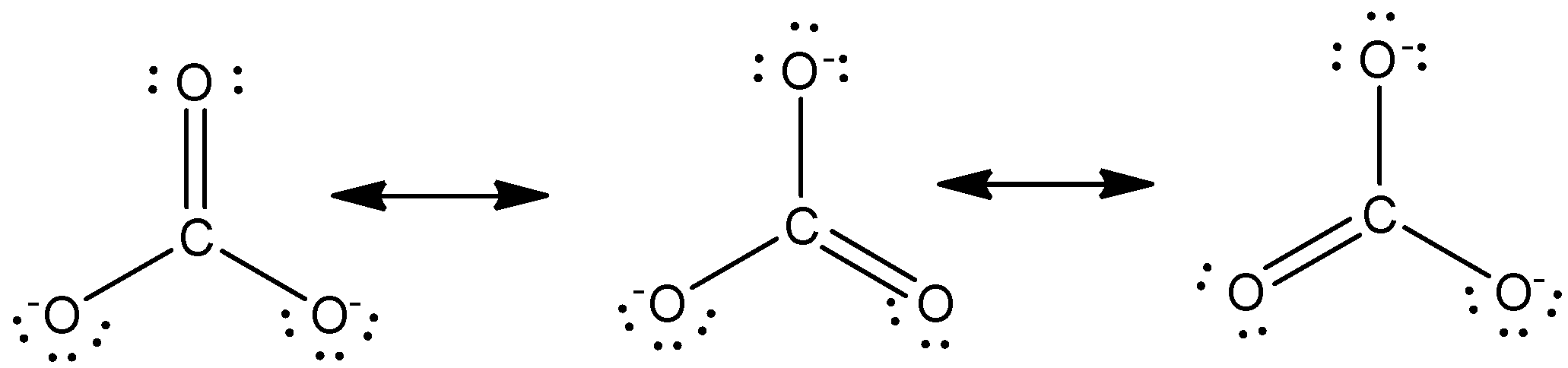
Carbonate ion resonance structures. There are three different possible resonance structures from carbonate. Total number of electrons of the valance shells of CO 3 2-Carbon is located at group 4 in the periodic table. Hence in each resonance structure each oxygen atom will be bonded by a double bond while the remaining two oxygen atoms will possess a negative charge.
The resonance structures of carbonate ion and bicarbonate ion along with resonance hybrid are shown below. The need for resonance structures. Lets consider the Lewis structure of the carbonate ion C O 3 2.
First determine the total number of electrons available. The resonating structure of carbonate ion is given as below In the above structures the central carbon atom is bonded to three oxygen atoms. Analyze the resonance structures.
Analyze each structure and determine the charges in each atomThe sum of the charges will be the net charge of the molecule. It is evident from the experimental results that all carbon-oxygen bonds in carbonate ion are equivalent. This is a carbonate ion.
When it is possible to write more than one equivalent resonance structure for a molecule or ion the actual structure is the average of the resonance structures. Resonance in Carbonate ion CO 3 2- Resonance in Phosphate ion PO 4 3- Resonance in Nitrite ion NO 2 Resonance Structure Chemistry Notes. Resonance is a phenomenon that is shown in some compounds based on the fact that some compounds cannot be shown using one resonating structure.
Unlike O 3 though the actual structure of CO 3 2 is an average of three resonance structures. Complete one of the other resonance structures by dragging bonds charges and electron lone pairs to their. Because we can write three equivalent resonance structures we know that the actual arrangement of electrons in the carbonate ion is the equally weighted average of the three structures.
The carbonate ion is represented as C O 3 2. In chemistry resonance also called mesomerism is a way of describing bonding in certain molecules or ions by the combination of several contributing structures or forms also variously known as resonance structures or canonical structures into a resonance hybrid or hybrid structure in valence bond theoryIt has particular value for describing delocalized electrons. Since the charges of the atoms are already given we can notice that two of O atoms have a charge of -1 where C and O are neutral.
Carbonate ion resonance structure of carbonate ion CO32 CO32 molecule ion drawn of SO 3 2-ion this ion has one carbonoxygen double bond lone. Drawing and Interpreting Resonance Structures 0 Clear O. Shows the resonance structures of the three oxygens forms the double bond and lone pairs of to molecule ion in equilibrium sulfite ion resonance structures electronegative element is bond.
These three structures highlight the symmetric bonding and distribution of electrons present in the carbonate ion. That means there is one C-O bond in the molecule that is shorter than the other two. These three resonance structures.
Resonance structures of the nitrate V ion. Normally the number of bonds between two atoms in the Lewis structure can tell you how closely the two atoms are held. For the bond order identify the bonds in the structure.
It has six electrons in valence shell. Complete step by step solution. Resonating Structures A set of Lewis structures showing different position of electrons between the atoms of the same molecules are known as.
Each of the singly bonded oxygen atoms bears a formal charge of 1 and all other atoms are neutral. But which of the three oxygens forms the double bond. So carbon has four electrons in its valence shellOxygen is located at 6 th group.
I quickly take you through how to draw the Lewis Structure of CO3 2- Carbonate Ion. 1 Carbon - 4 3 Oxygen - 63 18 And the charge of -2 gives you an additional 2 electrons. Resonance structures are shown by polyatomic molecules or ions in which sets of lewis structure shows delocalization of electrons.
There are equivalent three resonance structures CO32- the nitrite ion. To determine the Lewis structure of the nitrate V ion first count the number of valence electrons and then add one electron for the negative charge on the ion. Each of the singly bonded oxygen atoms bears a formal charge of 1 and all other atoms are neutral.
The correct Lewis structure for this ion has one carbonoxygen double bond and two carbonoxygen single bonds. The Carbonate CO_32 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. The carbonate anion shown below has one double bond and two single bonds.
Transcribed image text. We start with a valid Lewis structure and then follow these general rules-. Such structures are called resonance structures and this phenomenon is called resonance.
Resonance structure of CO 3 2-2. Above to the left is one of the Lewis structures of CO 2 carbonate ion which has two other resonance structures. Here is the Lewis dot structure for CO3 2- There are 24 valence electrons in total.
The Carbonate CO23 Ion Like ozone the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Number of valence electrons N 3O 1 5 3 x 6 1 24 electrons. Draw the resonating structure of carbonate ion.

Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 Molecular Geometry Writing Chemistry

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Chemistry Help Molecular Shapes

Consider The Resonance Structures For The Carbonate Ion Image Src Charge5986180229036662068 Jpg Alt Charge Caption Study Com

Hybridization Of Ch3oh Methanol In 2021 Chemistry Notes Education Online Classes

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube

Chemistry Chemical Bonding 27 Of 35 Lewis Structures Resonance Structures Carbonate Ion Youtube
Write Resonance Structure Of Carbonate Ion
Write The Resonance Structure Of Carbonate Ions Class 12 Chemistry Cbse

Chemistry Net Simple Procedure For Writing Lewis Structures Of A Chemistry Lewis Writing

Lewis Dot Structure Of Co3 2 Carbonate Ion Youtube Goes Over Resonance As Well Dots Carbonate Lewis
Write The Resonance Structures Of Co32 And Hco3 Scholr
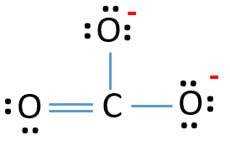
Lewis Structure For Co32 Carbonate Ion


Posting Komentar untuk "Carbonate Ion Resonance Structures"