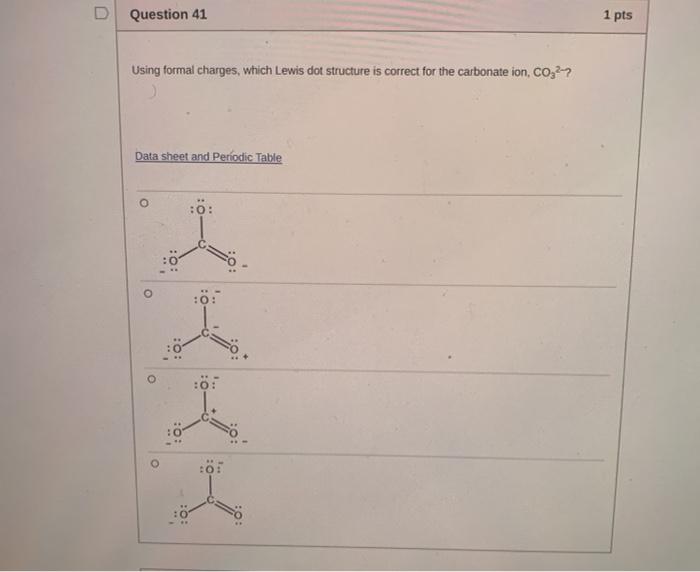
Carbonate Formal Charge
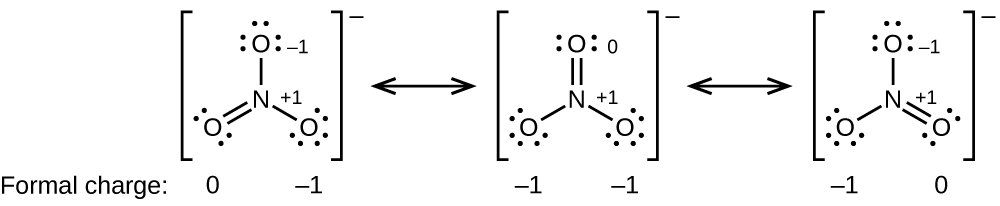
In the left resonance structure all the atoms have zero formal charge while on the right structure the nitrogen has a 1 formal charge and the oxygen with the single bond has a -1 formal charge. Now how is this so.
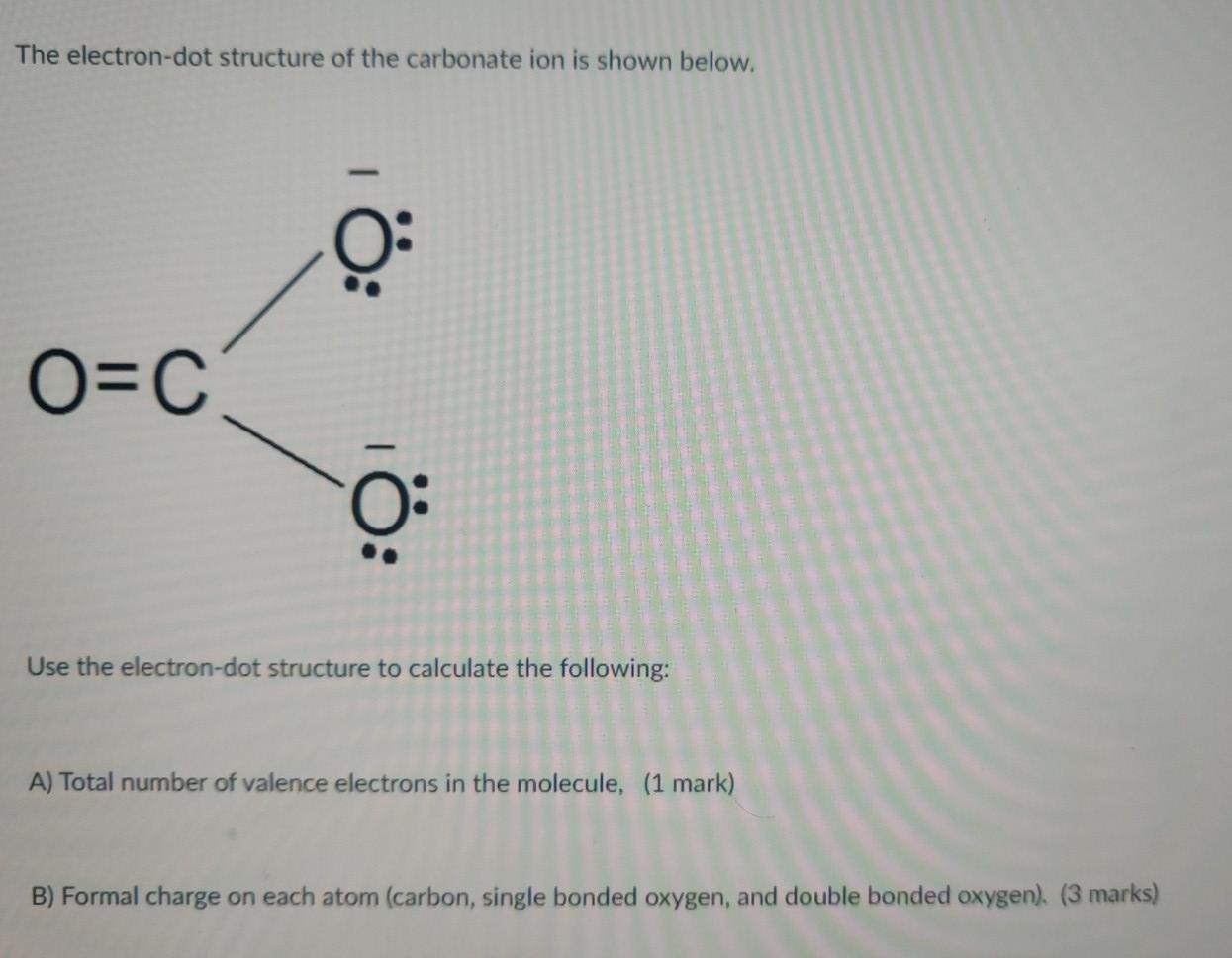
Solved The Electron Dot Structure Of The Carbonate Ion Is Chegg Com
Formal charge on carbon 4 0 8 2 0 The formal charge of any atom in a molecule can be calculated by the following equation.
Carbonate formal charge. How did it get an octet with only three bonds. One of the tools that we will eventually use to understand reactivity is formal charge. Since it gives one of its electrons to carbon it has formal charge 1.
NCI Thesaurus NCIt Hydrogencarbonate is the carbon oxoanion resulting from the removal of a proton from carbonic acid. All of the resonance structures for carbonate. F C V N 2 B Was this answer helpful.
The ion has two units of negative charge as there are two more electrons 32 than protons 30. It has a role as a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite a mouse metabolite and a cofactor. Formal charge of carbonate Structure Reactivity in Chemistry Introduction to Molecules IM5.
Lewis and Formal Charges Looking at the structure of a molecule can help us to understand or to predict the behaviour of that compound. Three carbon atoms now have an octet configuration and a formal charge of 1 while three carbon atoms have only 6 electrons and a formal charge of 1. Carbon monoxide dipole moment 0122 D.
The electronegativity for carbonate. The ionization energy for carbonate. Each oxygen has a formal charge of 23 and the carbon has a formal charge of zero.
It is a conjugate base of a hydrogencarbonate. The bicarbonate ion carries a negative one formal charge and is the conjugate base of carbonic acid H2CO3. Carbon has normal valence four but here it is only making three bonds even though it has an octet.
Using the structure of carbonate ion calculate the formal charge of a central carbon atom. Salts or ions of the theoretical carbonic acid containing the radical CO2 3. The formal charge on carbon atom in carbonate ion is.
It got an extra electron from somewhere the oxygen. Carbonate ion CO32- is a polyatomic anion with 1 carbon atom and 3 oxygen atoms. What is the formal charge on C atom in the carbonate anion.
The formal charges in the Lewis structure of CO do give you a hand-waving explanation for the fact that its not nearly as polar as formaldehyde which has no formal charges. So carbons supposed to have four valence electrons it has only three around it so it lost one of its electrons which gives it a formal charge of plus one. Lets look at two resonance structures of NO2.
Oxygen ions have a 2- charge so 3 of them would equal 6-. It has formal charge. So four minus three is equal to plus one so carbon has a formal charge of plus one.
It has a molecular mass of 6001 gmol and carries a total formal charge of 2. Since carbon has 4 valence electrons its formal charge will be zero. CO 2 3 2 H 2 O HCO 3 H 2 O OH H 2 CO 3 2 OH.
The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. What is the formal charge of no2. The formal charge on the central xenon atom in.
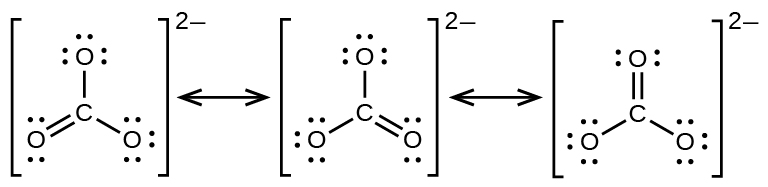
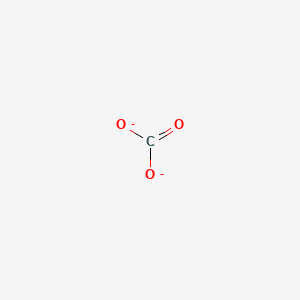
We can convert each lone pair to a bonding electron pair which gives each atom an octet of electrons and a formal charge of 0 by making three CC double bonds. Metal carbonate compounds are common in. Carbonate ion CO 3 2-Carbonate ion has a -2 charge.
Then what is the structure of carbonate ion. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. And the conjugate acid of CO 2 3 the carbonate ion as shown by these equilibrium reactions.
In order to calculate the formal charges for CO32- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e. It is both the conjugate base of carbonic acid H 2 CO 3. It can be said that the carbonate ion has a charge of 2 and each of the single bonded oxygen atoms holds a charge of 1.
You will learn about these facts in this tutorial. Carbonate Ion is a polyatomic ion with formula of CO3 2-. Bicarbonate Ion is a polyatomic ion whose formula is HCO3-.
The formal charge on each atom in carbonate. The carbonate ion is the simplest oxocarbon anion consisting of one carbon atom surrounded by three oxygen atoms. After finishing the lewis structure of CO 3 2- there should be a -2 charge and it should be stabile structure.
8 4 Formal Charge Chemistry Libretexts
Solved D Question 41 1 Pts Using Formal Charges Which Lewis Chegg Com
Ammonium Sulfate Nh4 2so4 Pubchem

1 Write Formal Charges Of The Atoms I In The Carbonate Ion And Ii In The Nitrite Ion
7 4 Formal Charges And Resonance Chemistry

7 4 Formal Charges And Resonance Chemistry

1 Write Formal Charges Of The Atoms I In The Carbonate Ion And Ii In The Nitrite Ion

In Bisulphate Ion The Formal Charge On Sulphur Atom Is
Phosphonium Tributylmethyl Carbonate 1 1 C14h31o3p Pubchem
If Carbon Has 4 Valency And Oxygen Has 2 Valency Then How Does It Combine To Form Carbonate Quora
Chemistry Net 01 01 2012 02 01 2012

The Best 13 Formal Charges Of Co32 Datul Imaliya
Nickel Ii Carbonate Cnio3 Chemspider

Posting Komentar untuk "Carbonate Formal Charge"