Carbonate Bond Length
Bond order in CO2 2 bond order in CO 3 and bond order in CO32- in between 1 and 2 or 15 now greater the bond order shorter the length. So in order of longest to shotest.
Secondly what is the bond order of carbonate.
Carbonate bond length. Bond length is inversely proportional to bond order. Given the following tabulated values of bond energy and bond length. Analyze the bonds in the carbonate ion.
What is average C-O bond order bond length and bond energy. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. Referring to the table above a double bond between carbon and oxygen has a bond length of approximately 67 57 124 pm and a triple bond between carbon and oxygen has a bond length of approximately 60 53 113 pm.
It is a conjugate base of a carbonic acid. It is a conjugate base of a hydrogencarbonate. Hydrogencarbonate is the carbon oxoanion resulting from the removal of a proton from carbonic acid.
In certain molecules axial and equatorial positions are unique. In addition to the single. For the best answers search on this site httpsshorturlimawLTr.
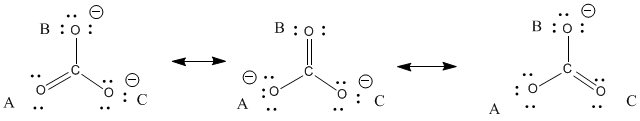
Bond Length pm Enthalpy kJmol Bond Length pm Enthalpy kJmol Bond Length pm Enthalpy kJmol Bond Length pm Enthalpy kJmol H-H 74 436 N-S 168 Si-Br 216 Cl-Cl 199 242 H-F 92 567 N-F 139 272 Si-I 240 Cl-Br 214 218 H-Cl. Hope this helps Mark as brainliest. Applying this same method to the carbonate ion we have 3 resonance structures with bond orders of 2 1 1 when considering the bond between carbon and a single oxygen.
Why are C-O bond lengths in a carbonate ion identical. All of the bonds in the carbonate ion Co_3_2- are indentical same bond orderlength and energy. Bond Bond Energy kJmol Bond Length pm C-O 358 143.
What do you infer about the CO bonds in the carbonate ion. Molecules have a balanced geometric shape the bonds have a certain length and angle as well and the laws of quantum mechanics determine this. Since there are 3 structures the answer is 2113133333 or 43.
Bond Lengths and Enthalpies. Instead you can use resonance structures to understand this fact. It has a role as a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite a mouse metabolite and a cofactor.
Pdf file 35mb - high resolution JPG file Size. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. The bond order for the carbon-oxygen bond in carbonate is 133.
The partial double bond comes from delocalized pi bonding. The length of the bond is determined by the number of. Therefore the bond length is greater in CO 2.
Specifically compare the standard bond lengths for C- and CO in Tables A and 2 with the WebMO-computed bond length Table 3. Use bond angles in PCls to identity and describe. Answer Lewis diagram for carbonate ion is.
One of the 3 oxygens is enaged in the double bondand the other two oxygens connected to craboon by a single sigma bond each hold the negative charge of one electron excess each. Bond order depends on the number of bonds and bond length is inversely proportional to the bond order. Thus the carbonate ion has the longest bond length followed by carbon dioxide and finally carbon monoxide.
Hence the bond length order will be as mentioned above. The bond length of all three C-O bond in carbonate ion is 136 pm. The carbonate ion has three resonatance structures which result in.
Bicarbonate Ion is a polyatomic ion whose formula is HCO3-. Compare Calculated Bonds for O-C. In CO32- the bond lies between single and double bond.
CO triple bond 1072 109. Co Bond Length. The bond order is 2 not 4 for ceCO2 and the bond order for the carbonate ion is somewhere between 1 and 2 due to resonance.
CO2 has double bond OCO due to its linear structure with carbon it can have 2 double bond with oxygen. In carbonate ion all the three C -O bonds have identical bond length. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
The carbonate ion according to its Lewis di agram has two types of carbon -oxygen bonds one double bond and two single bon ds suggesting that one carbon. You cannot draw a Lewis structure that would suggest all three bonds are the same length. Here CO has a triple bond.
Bond order of c-o in CO CO2 and Co3-2 is 32133 respectively. Carbonate ion is a symmetric trigonal planar molecule. How is carbon oxygen bond length determined.
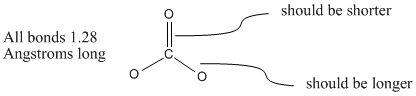
The single double and triple bond lengths of carbon in carbon dioxide are respectively. All three carbon-oxygen bond distances are about 128 Angstroms long. The bond length of all three C-O bond in carbonate ion is 136 pm.
Carbonates are readily decomposed by acids. The carbon in Carbonate ion is in an sp2 hybridisation and there are 3 sigma bonds and one pi bond around it. TPSSh6-31G2dfp Histogram of Bond lengths in Å vs number of species Values greater than 230 are in the 230 bin.
Why does co3 2 have identical bond lengths. The chemical equation and the structural equation of a molecule are the two most important factors in.

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram
Bond Length Definition Formula Calculation Video Lesson Transcript Study Com

Jgg Thesis 01 Surface How To Apply Algorithm

Click On Image To Zoom Calcium Carbonate Calcium Crystals

A The Dft Structure Of The Isolated Hydrogen Carbonate The Symmetry Download Scientific Diagram

Calcium Carbonate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

6 Step Organic Chemistry Reaction Identification Functional Group Organic Chemistry Reactions Problem Solving

Convertible Bonds Bond Finance Investing

All The C O Bonds In Carbonate Ion Co3 2 Are Equal In Length Explain
Posting Komentar untuk "Carbonate Bond Length"