Sodium Carbonate N Factor
20 ml of H 2 O 2 after acidification with dil. N factor of Ca OH22.

Type Of Reaction For Na2co3 Hcl Nacl H2o Co2 Youtube
23 2 12 16 3 46 12 48.

Sodium carbonate n factor. 132 Alternatively calculate the alkalinity as sodium car-bonate as follows. Once you realize there are two sodium ions per carbonate ion the problem is simple. H 2 S O 4 required 30 ml of 1 2 N K M n O 4 for common oxidation.
Given in the question Normality of sodium carbonate 02 N The formulae that defines the relation between normality and molarity is normality is equal to the product of n. Na₂CO₃ So molecular weight 2 23 12 3 16 106 gmol Here we can observe. And sodium carbonate the model predicts the solubility well at 25 oC.
The strength of H 2 O 2 solution is Molar mass of H 2 O 2 3 4. Thus 02 N Na2CO3 022 M Na2CO3 01 M Na2CO3. Na₂CO₃ 2Na CO₃ Hence n - factor change in oxidation number 2 - 0 2 So equivalent weight molecule weightn-factor Molecular weight2 This way equivalent of Na₂CO₃ 1062 53.
Find an answer to your question n factor of sodium carbonate THERASABISHMA THERASABISHMA 30062018 Chemistry Secondary School answered N factor of sodium carbonate 1 See answer THERASABISHMA is waiting for your help. S with 1991 production of over 9 million megagrams Mg 102 million tons. The objectivity of the present study is to assess the difference between various forms of.
Now n-factor of HCl is 1 and that of Na2CO3 is 2. N-factor of a reaction is not defined but it is defined for a single species participating in that reaction. Reagent Chemical N Nitrogen liquid kg 043 Reagent Chemical Na2B4O710H2O Borax anhydrous powder kg 165 Reagent Chemical Na2CO3 Sodium carbonate caustic soda 50 Na2CO3 kg 50059 Reagent Chemical Na2Si3O7 Sodium silicate at 48 Na2Si3O7 kg 0071.
Or 01 mole X molecular mass of Na2CO3 01 mole X 10599 gL. N factor here we mean a conversion factor by which we divide molar mass of substance to get equivalent mass and it depends on nature of substance which varies from one condition to another condition. Thus 02 N Na2CO3 022 M Na2CO3 01 M Na2CO3.
10gm of a hydrated sodium carbonate. Now we will calculate the molar mass of sodium carbonate. Multiple Logistic Regression Analysis Showing sodium carbonate freshly mixed in relation with oral conditions.
Solubility of N 2 O in Na 2 CO 3-solutions at 25 o C 0 5000 10000 15000 20000 25000 30000 0 4 8 12162024. Sodium carbonate actual5 A 2 B 3 06308. This inorganic compound is water-soluble and when dissolved in water it forms carbonic acid and sodium hydroxide.
Find the normality of a solution prepared by dissolving 0321 g of sodium carbonate N a 2 C O 3 in 250 mL of water. N a 2 C O 3. XH 2O on strong heating loses a weight of 63gm.
The peptide antigen 1 nmol in sodium carbonate sodium bicarbonate buffer pH 96 is incubated at room temperature overnight in a 96-well microtiter plate. HERE IS YOUR ANSWER. Sodium carbonate Na2CO3 or CNa2O3 CID 10340 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
It should be noted that in the original paper of Weisenberger and Schumpe they mention that the model for the solubility of N 2O into carbonate solutions should be valid up to 40 oC. Add your answer and earn points. Or 01 mole X molecular mass of Na2CO3 01 mole X 10599 gL.
Over 85 percent of this soda ash originates in Wyoming with the remainder coming from Searles Valley California. N a 2 C O 3 HCl NaCl NaHC O 3. Sodium carbonate wt 5 17101 3 Na2O wt 2 133 If actual sodium carbonate content is desired the sodium bicarbonate content must be determined separately as described in Sections 16 and 23.
N factor of NaOH1. Normality N n factor X Molarity M n factor for salt Na2CO3 is the number of moles of electron involved by 1 mole of the salt. 812 Sodium Carbonate 8121 General1-3 Sodium carbonate Na2CO3 commonly referred to as soda ash is one of the largest-volume mineral products in the U.
N factor of B OH31 becau. 01 M Na2CO3 is 01 mole Na2CO3 L. Normality N n factor X Molarity M n factor for salt Na2CO3 is the number of moles of electron involved by 1 mole of the salt.
In its pure form it is white powder and odourless. Here there are 2 electron involved so the n factor for Na2CO3 is 2. N- Number of patients in a particular category TABLE 2- Showed number and percentage of snuff users with and without sodium carbonate in both forms that is frshly mixed and premixed.
On the basis of the above said logic the n factor for NaHCO3 will be 1 because it ionizes into sodium ion and bicarbonate ion and both ions possess unit. N factor for a salt is equal to total positive charges of cations or total negative charges of anions. To calculate the equivalent mass of sodium carbonate firstly we will write the chemical equation.
Here there are 2 electron involved so the n factor for Na2CO3 is 2. The plates are blocked by the addition of 1 bovine serum albumin in coating buffer incubated at 37C for 1 hr and washed with PBS containing 005. 01 M Na2CO3 is 01 mole Na2CO3 L.
N factor of Al OH33. Convert grams Na2CO3 to moles or moles Na2CO3 to grams. N 0321 g Na 2 CO 3 x 1 mol10599 g x 2 eq1 mol N 01886 eq02500 L N 00755 N.
It is a product of molaritynormality. The correct option is D 0024 N Equivalent weight of N a 2 C O 3 molar mass n-factor 106 2 53 Normality number of gram equivalents total volume of solution. Sodium Carbonate Formula.
To solve this problem you need to know the formula for sodium carbonate. There is an exchange of two electrons in sodium carbonate hence the n factor of sodium carbonate is 2. Molecular formula of sodium carbonate.
Sodium carbonate raise the pH of the snuff to 89 in order to facilitate nicotine absorption through the oral mucous membranes. It is also known as Soda crystals soda ash washing soda. Sodium carbonate is a diazonium salt of carbonic acid with chemical formula Na2CO3.
IS SODIUM-CARBONATE IN SNUFF A CAUSATIVE FACTOR FOR ORAL MUCOSAL LESIONS A CROSSSECTIONAL ANALYSIS Abstract.

Type Of Reaction For Na2co3 Hcl Nacl H2o Co2 Youtube

In A Reaction 5 3 G Of Sodium Carbonate Reacted With 6 G Of Acetic Acid The Products Were 2 2 Youtube

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube

How To Balance Na2co3 Na2o Co2 Sodium Carbonate Decomposing At High Temp Youtube
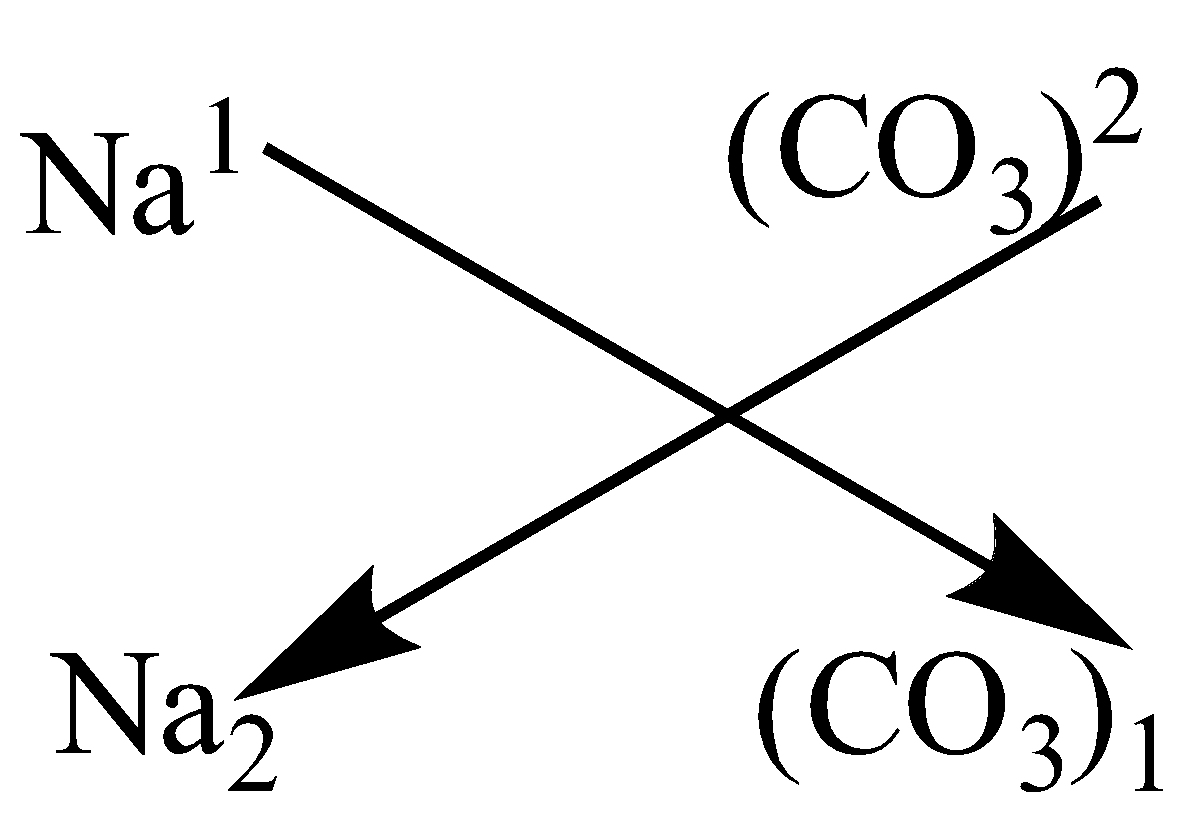
The Formula Of Sodium Carbonate Is Na2co3 Atrue Bf Class 10 Chemistry Cbse

Na2co3 Hcl Sodium Carbonate Hydrochloric Acid Youtube
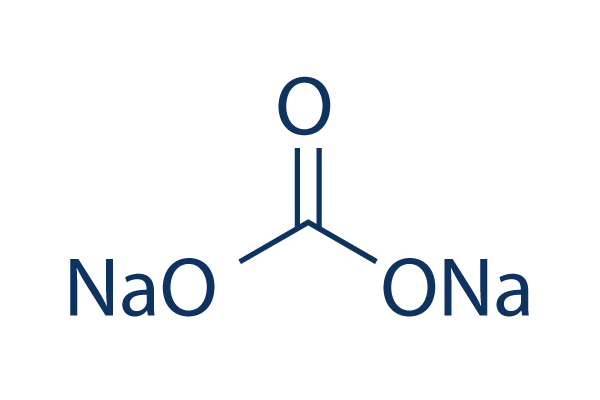
Sodium Carbonate 99 Hplc Selleck Others Chemical
Sodium Acetate Ch3coona Pubchem

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube
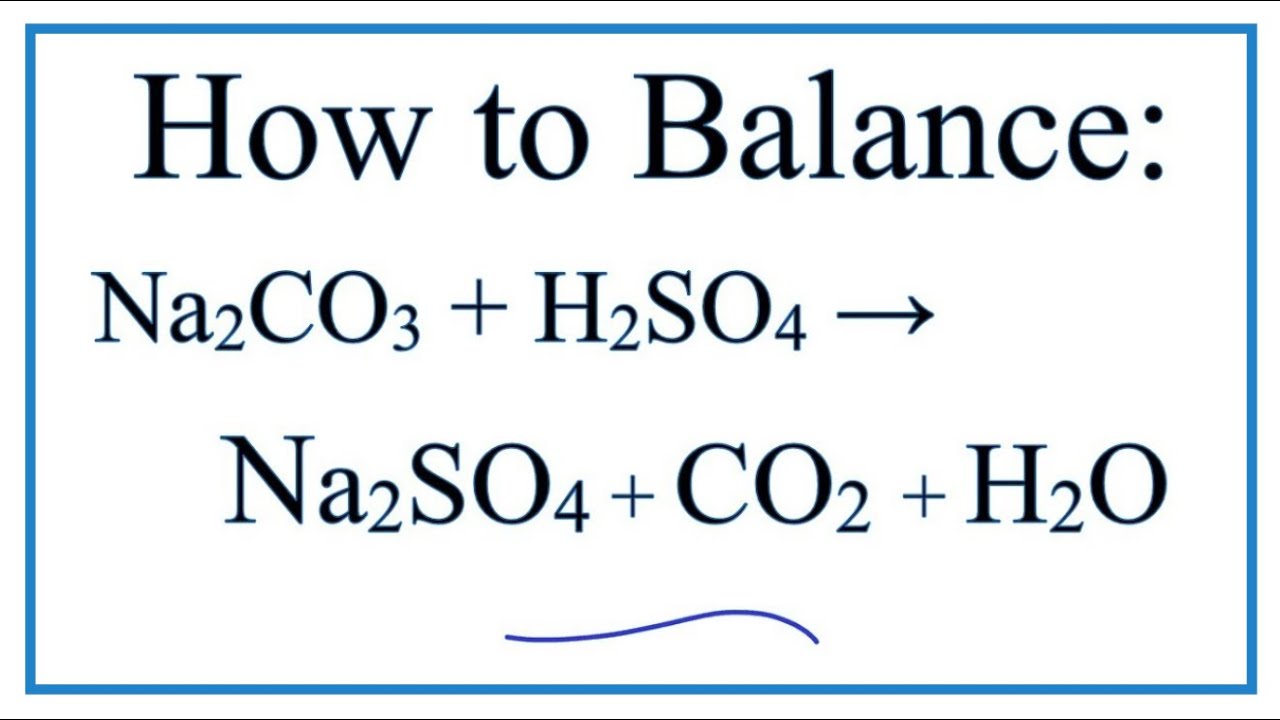
How To Balance H2so4 Na2co3 Na2so4 Co2 H2o Youtube

What Is The Normality Of 0 478 G Sodium Carbonate In A 100 Ml Solution Brainly In

Sodium Carbonate An Overview Sciencedirect Topics
Sodium Carbonate Na2co3 Pubchem


Posting Komentar untuk "Sodium Carbonate N Factor"