Carbonate Buffer Ph 7
2 Add sodium chloride to 014 M final concentration and 1 vv horse serum final. Carbonate Buffer pH 97.
Buffer Formulations Exalpha Biologicals Inc
Carbonate-Bicarbonate Buffer pH 92 to 106 preparation guide and recipe.

Carbonate buffer ph 7. Buffers are broadly divided into two types acidic and alkaline buffer solutions. A mixture of sodium bicarbonate and sodium carbonate is a buffer and can be used between pH of about 88 to 106 at 37 o C or about 92 to 108 at 20 o C. There are other uses but thats general knowledge of chemical solutions not a physiological phenomenon or system.
Dissolve 84 g of sodium bicarbonate and 106 g of sodium carbonate in sufficient water to produce 500 ml. Well IIRC carbonate is only used to buffer above 80 and. Recipe can be automatically scaled by entering desired final volume.
Rogers Thu 11 Jun 1992 CO2 systems and fresh water plants. Buffer Preparation Formulas and Equations. The other 1 is dissolved Carbon Dioxide.
API PROPER pH 82 contains bicarbonate and carbonate buffers and will directly increase carbonate hardness. I would be at least a little worried about the effects of running. Use ammonium bicarbonate reagent to avoid the carbamate impurity present in most ammmonium carbonate.
The bicarbonate buffer system is what the body uses. You may find that you can get by with lower concentrations of ammonium bicarbonate which is a good buffer in the range from 64 to 74. A simple phosphate buffer is used ubiquitously in biological experiments as it can be adapted to a variety of pH levels including isotonic.
For H2CO3Ka1 47 10-7. The KH level is also a measurement of waters ability to resist pH change. If I know the second dissociation constant of phosphoric acid I could figure out exactly how to make a pH 7 standard 01 M phosphate buffer solution using phosphate salts.
PKa1 since below that pH you are in equilibrium with H2CO3 which can. Bicarbonate buffer mostly have a pH close to 74. Ka2 47 10-11.
It is the major small intestine buffer. This means that although. Sodium Carbonate Sodium Bicarbonate Buffer Preparation pH 92-108.
If the pH of 1 L of a 10 M carbonate buffer is 70 what is the actual number of moles of H2CO3 and HCO3-. Notice that there is a gap in buffering between pH 31 and pH 62 for phosphate. The carbonate and phosphate buffer systemsthese are not in the pH range necessary to buffer blood.
API PROPER pH 65 70 and 75 contain no carbonate hardness but will cause a spike in KH as measured with KH test kits. Sodium Acetate Acetic Acid Buffer Preparation pH 37-56. Preparation of Bicarbonate-Carbonate Buffer Solutions pH 9121083 To create 100ml of a 01M bicarbonate buffer solution mix sodium bicarbonate and sodium carbonate decahydrate as.
From the calculation above the pH of buffer solution is 738. Imidazole glyoxaline HCl Buffer Preparation pH 62-78 at 25 C. This question now b.
Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings. Carbonate-Bicarbonate Buffer pH 92 to 106 Preparation Commonly used for various immunoassay applications and for many protein and antibody conjugation procedures including sandwich ELISA which require experimental surface coatings. Click hereto get an answer to your question An enviromental chemist needs a carbonate buffer of pH 10 to study the effects of the acidification of limestone - rich soilsHow many grams of Na2CO3 must be added to 15 L of freshly prepared 02 M NaHCO3 to make the buffer.
Changes in hydrogen carbonate ion concentration however require hours through the relatively slow elimination through the kidneys. Now lets check our answer to see whether its reasonable. Chloride Buffer pH 20.
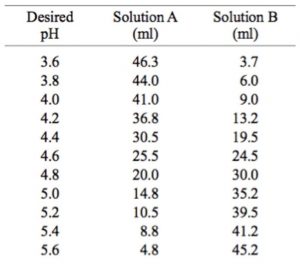
For reaching 85 you will need a large amount of bicarbonate and very small amount of carbonate. For example a mixture of acetic acid and sodium acetate acts. The Carbonate Buffer Contents.
What this means is that this system is. Carbonic acid concentration is controlled by respiration that is through the lungs. 005 M succinic acid 59 gl Sodium chloride Horse serum METHOD 1 Take 1000 ml of A and add B until pH is 75.
Acidic buffers are solutions that have a pH below 7 and contain a weak acid and one of its salts. 005 M sodium tetraborate Na 2B 4O 710H 2O 190 gl Solution B. Recipe can be automatically scaled by entering desired final volume.
The balanced equation is below. Na 2 HPO 4 NaH 2 PO 4 Buffer Preparation pH 58-80 at 25 C. The buffer through a vacuum filter unless your pH is well above 74 1st.
The carbonic acid-hydrogen carbonate ion buffer works throughout the body to maintain the pH of blood plasma close to 74. By signing up youll get. Phosphate Buffer pH 58 to 74 preparation guide and recipe.
Dissolve 657 g of potassium chloride in water add 1190 ml of 01. The bicarbonate acts like a buffer in the ocean. 62.
015 M boratesuccinate buffer pH 75 for tanning erythrocytes MATERIALS Solution A. For instance if the concentration of HCO 3 was equal to the concentration of H 2 CO 3 then the concentration of H 3 O will be equal to the acid dissociation constant K a. Note that the bicarbonate-carbonate buffer works best in the pH range 92-108.
As you probably noticed the chemical reaction has multiple steps and can go in both directions. Practical limits of column stability require that we truncate the lower range to 20.

Phosphate Buffer Saline With Tween Pbst Biomat

Effect Of Ph On Activity With Hydrogen Carbonate And Formate Download Scientific Diagram

How Do I Prepare Carbonate Buffer

Phosphate Buffer Saline Pbs Biomat

How Do I Prepare Carbonate Buffer

Preparation Of Buffer Solutions Pharmaceutical Guidelines

How To Calculate The Hco3 H2co3 Buffer Ratio In Blood Youtube

Bio 98 Lecture 2 Acid Base Equilibria Ph And Buffers Ppt Download

Phosphate Buffer Ph 7 0 0 5 M Cepham Life Sciences Research Products
Lecture Goals To Review How Ph And Alkalinity Work Ppt Video Online Download

Acid And Base Balance Department Of Biochemistry 1

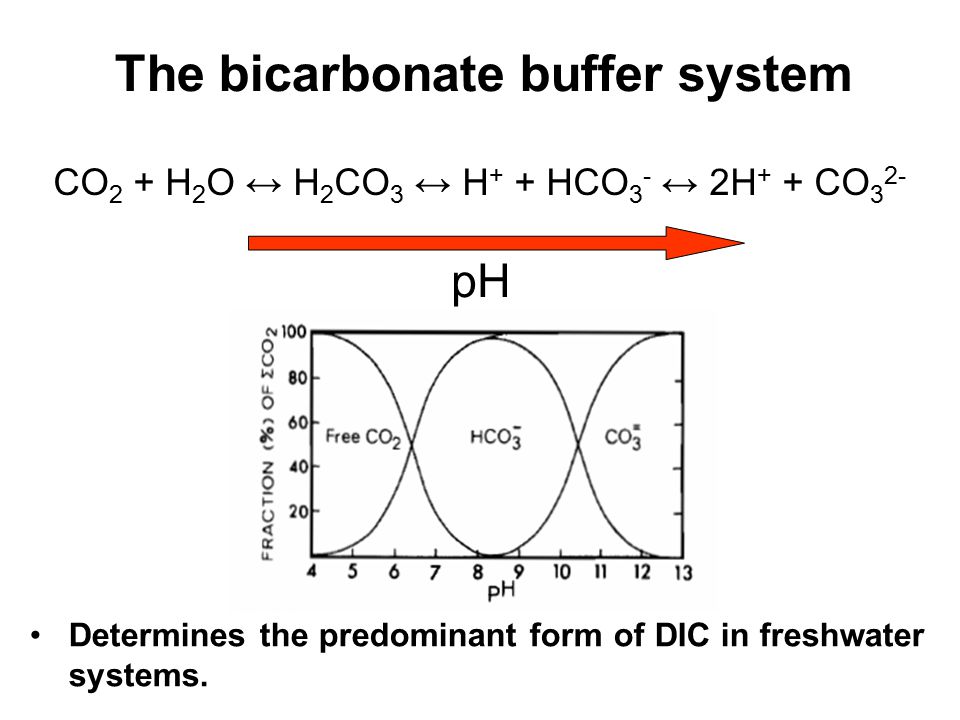
Relationship Between Bicarbonate Buffer System And Carbon Dioxide Download Scientific Diagram

Posting Komentar untuk "Carbonate Buffer Ph 7"