Carbonate Qualitative Analysis Reaction
In one part we will test for sulphite and in the other part for thiosulphate. HCl gives CO 2 gas that reacts with lime water to produce a white precipitate of calcium carbonate that turns lime water milky.
Separation And Qualitative Analysis Of Carbonate Phosphate And Chromate Anions In Qualitative Inorganic Courses
Strongly acidic pH10.

Carbonate qualitative analysis reaction. CO 3 2. Bubble gas through limewater to test for CO2 will turn limewater cloudy 2HCl Na2CO3 2NaCl H2O CO2 Testing for presence of a sulfate. For the confirmatory analysis of acidic radicals we use the soda extract or sodium carbonate extract.
Take part 1 and add 05ml fuchsin reagent magenta colored dye. Test Results Solution Type Indicator Acetic Acid Carbonate lon Observations. Obtain exposure to deductive reasoning as a scientific approach.
H 2 SO 4 but they give ppt. When reacting with barium chloride solution. Bubble gas through limewater to test for CO 2 will turn limewater cloudy 2HCl Na 2CO 3 2NaCl H 2O CO 2 Testing for Presence of a sulphate.
In qualitative analysis the given compound is analyzed for the radicals ie cation and the anion that it contains. We will also discuss preliminary test for carbonate and bicarbonate. Unknown Analysis of the Answer Sheet.
Acetic and boil to expel CO 2. Preparation of sodium carbonate extract. The qualitative inorganic analysis is a method of analytical chemistry that seeks to find out the elemental composition of inorganic compounds through various reagents.
Aqueous sodium carbonate and sulfuric acid react with the evolution of a. Carbonate on reaction with dil. Phosphates PO 4 3.
A qualitative analysis of ions on a test-tube scale. This type of reactivity can be extended to the base carbonate CO 3 2- which can react with an acidic solution causing the formation of carbon dioxide bubbles due to the. To prepare the sodium carbonate extract take 13 ratio of unknown salt and sodium carbonate in 15text ml of water ie.
Nitrate gives a corresponding reaction only when conc. Barium chloride solution group. If heated indirectly not burned magnesium reacts with oxygen in air to form white magnesium oxide.
Part 1 Introduction to chemical tests. CO 32- CO 2 H 2 O 2HCO3-. Sodium Bicarbonate 3 4 acid Acetic Acid 3 3 acid Table 3.
In case of. The carbonate ion is the anion of carbonic acid which is a weak acidSome compounds of carbonate ion forms precipitates and some metal carbonates are soluble in waterAlso some metal carbonates have colours in. 314 Qualitative analysis Fizzing due to CO 2 would be observed if a carbonate was present Testing for Presence of a carbonate Add any dilute acid and observe effervescence.
Carbonate Reactions CO3 2- A1. Of barium phosphate soluble in Dil HCl. Divide this colorless solution in two parts.
Qualitative analysis of anions is not as systematic as cations. What is Qualitative analysis. Qualitative analyses are mainly used for the determination of anions and cations present in the given sample.
For each unknown. In the qualitative analysis procedure the chemical properties of an unknown substance are determined by systematically reacting the unknown with a number of different reagents. It is prepared when the given inorganic compound is insoluble in water.
Sulphuric acid is added in place of dil. Tests for carbonate ion compounds reactions precipitates. HCl gives CO 2 gas that reacts with lime water to produce a white precipitate of calcium carbonate that turns lime water milky.
Physical procedures like noting the colour smell or taste of the substance have. Salt solution barium chloride solution a white ppt. Qualitative and quantitative analysis of microplastics by Py-GCMS.
Part 2 Qualitative tests to identify organic molecule functional groups of homologous series Part 3 Metal cations positive ions metal carbonates ammonium ion and hydrogen ions acids this page. In case of soluble carbonate this test is performed with water extract and in case of insoluble carbonates this test. Confirmatory tests for carbonate in presence of bicarbonate and sulphite is also discussed in this post.
If the solution gets decolorized it. Abstract Environmental pollution by microplastics MPs has attracted much attention due to possible risks of MPs to human health and fast and reliable analytical methods are required for identification and quantification of MPs in various matrices. Part 4 Gases water and nonmetallic elements Part 5 Anions negative ions including hydroxide alkalis.
By predetermining what the particular reaction will produce if a specific ion is present the ions that actually are in. Chemistry questions and answers. No change thermally stable.
314 Qualitative analysis Fizzing due to CO2 would be observed if a carbonate was present Testing for Presence of a carbonate Add any dilute acid and observe effervescence. The unknowns will be sodium salts of the following anions. Qualitative Analysis When nitric acid reacts with another chemical the resulting salt will be a nitrate and all nitrates are soluble in water.
Chemistry Qualitative Analysis Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers Analytical chemistry deals with qualitative and quantitative analysis of the substances. Reaction with dil HCl. Carbonate on reaction with dil.
This test is based on the fact that the anions of this group do not react with dil HCl or conc. What is Qualitative Inorganic Analysis. In contrast to the cation qualitative analysis schemes done previously the unknowns here will be solid samples rather than solutions and each sample will consist of a single salt rather than a mixture.
For qualitative analysis it is useful to divide the pH scale into at least three categories. To this add a few drops of m-phenylenediamine hydrochloride solution followed by a few drops of hydrochloric acid. Processes and techniques needed to identify the following ions in an unknown compound.
Become an expert at writing net ionic equations. If burned magnesium burns with an intense white flame to give magnesium oxide. Carbonate nitrate nitrite phosphate sulfate and sulfite.
QUALITATIVE ANALYSIS OF ANIONS ----- LEARNING GOALS 1. This analysis is a type of analytical chemistry that is used to determine the elemental composition present in the inorganic compounds via different reagents. This post is about how we can categorize them in groups.
QUALITATIVE ANALYSIS OF REACTIONS Data Sheet Table 2. Alternatively imagine using hydrochloric acid to acidify a reagent before adding silver nitrate. 2 Na 3 PO 4 3BaCl 2 Ba 3 PO 4 2 6 NaCl.
2Mgs O 2 g 2MgOs h. Unit 92 Qualitative analysis - Lesson 3 of 3 Page 8 of 8 g. Carbonate on reaction with dil.
Acidify 1-2 mL of the sodium carbonate extract with dil. Consequently the formation of misleading erroneous precipitates will be avoided. PH Paper Test Results of OH and H Solution Type Initial Litmus Color Final Litmus Color Acid or Base.

Testing For Cations Qualitative Analysis Calcium Ca 2

Chm 212 Experiment 3 Analysis Of A Mixture Of Carbonate And

Derivatographic Analysis Of Basic Zinc Carbonate A Tg Curve B Dtg Download Scientific Diagram
Lesson Explainer Further Tests For Anions Nagwa
Separation And Qualitative Analysis Of Carbonate Phosphate And Chromate Anions In Qualitative Inorganic Courses

Qualitative Analysis Of Metal Cations Chemistry High School Chemistry Analysis

Colour Chemistry With D Block Quantum Chemistry Chemistry Classroom Chemistry Education Teaching Chemistry

Pin On Coool Steampunk Machanisms

Chemistry Salt Analysis Class 12 In 2021 Chemistry Chemistry Basics Science Notes
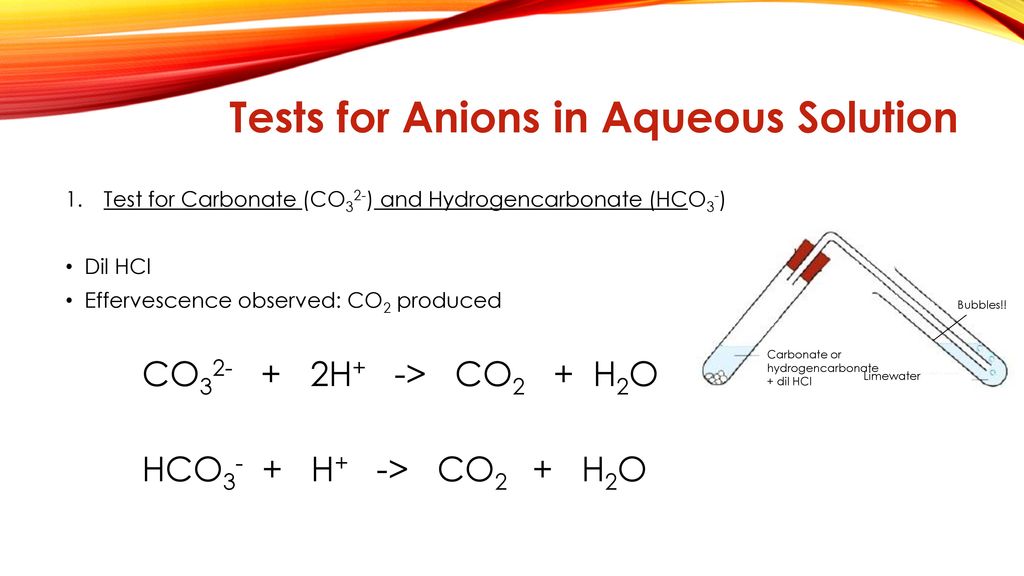
6 Chemical Equations Tests For Anions Ppt Download
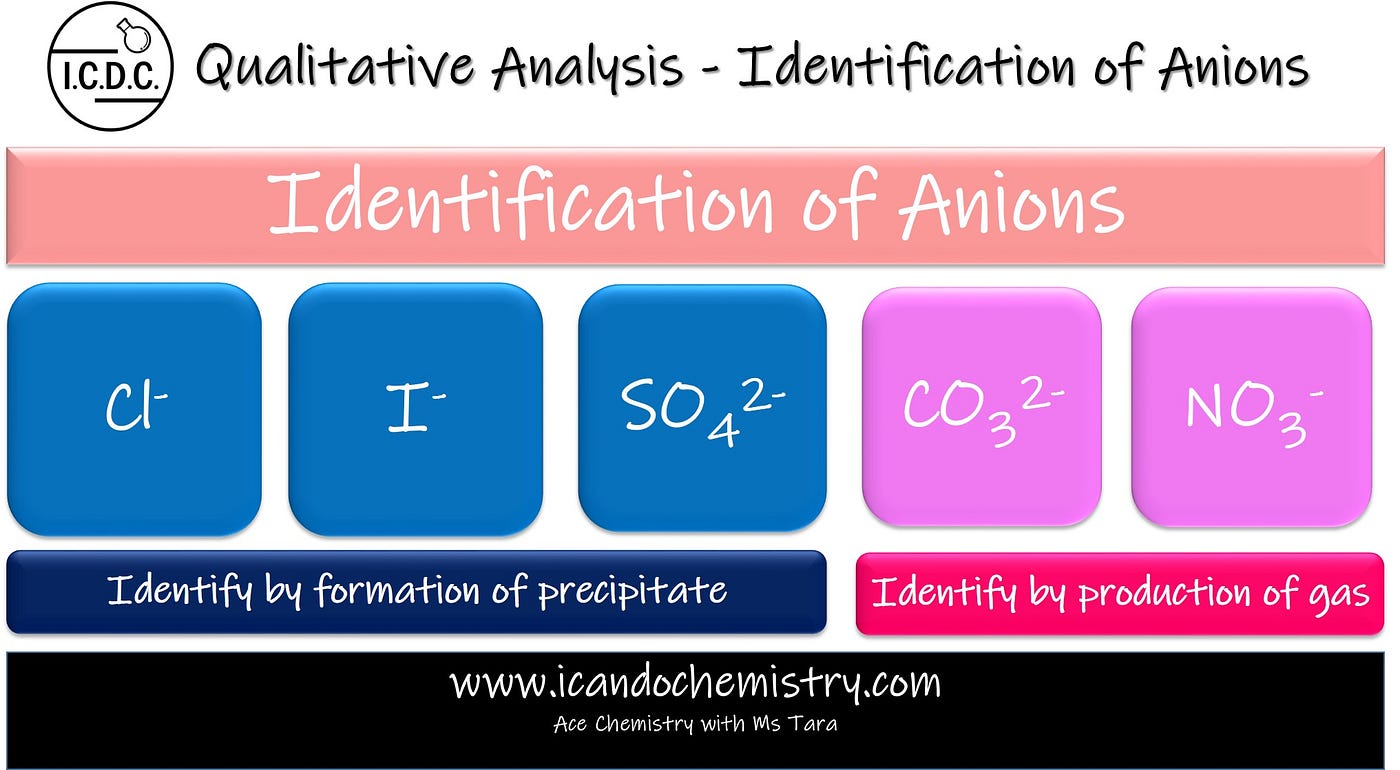
Qualitative Analysis Identification Of Anions By I Can Do Chemistry Tara Puah Medium
Lesson Explainer Further Tests For Anions Nagwa
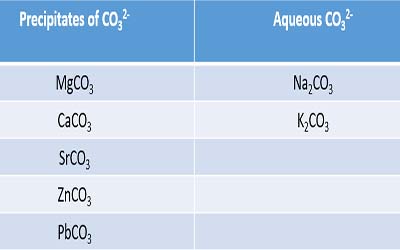
Tests For Carbonate Ion Compounds Reactions Precipitates



Posting Komentar untuk "Carbonate Qualitative Analysis Reaction"