Carbonate Bicarbonate Titration With Hcl
You will need about. Answer 1 of 3.

Titration Of Sodium Carbonate With Hydrochloric Acid Youtube
Titration method phenolphthalein indicator 25ml of carbonatebicarbonate mixture was pipette into a conical flask and two drops of phenolphthalein was added the solution changed from colourless to pink.

Carbonate bicarbonate titration with hcl. Na 2 CO 3 HCl NaCl NaHCO 3. The total amount of carbonate and bicarbonate in the mixture is determined by titration against standard hydrochloric acid using methyl orange as indicator. Table 1 shows the value that was obtained.
Volume NaOH added for bicarb content. Aqueous sodium carbonate titration with HCl solution. Volume HCl delivered for alkaline total first titration.
Another titration using phenolphthalein as indicator will give the concentration of carbonate. Volume_hcl Volume Of Sodium Hydroxide Volume Of Sodium Carbonate2 Go. Titration to the bromocresol green end-point ensures that all of the carbonate and bicarbonate have been converted to H 2CO 3 and then to H 2O and CO 2.
1M HCl until the pink colour changed to colourless. Your best bet is to begin with sodium bicarbonate and heat it to produce your own fresh carbonate. Before asking a question it is often useful to carry out a search first because questions have frequently been answered in the past - just like this one Why do we heat the mixture of calcium carbonate and hydrochloric acid in a.
HCO 3 aq H. Can sodium carbonate-sodium bicarbonate buffer be used for EDTA titration of calcium. Titration of Sodium Carbonate with Hydrochloric Acid.
Titration of bariumII hydroxide by hydrochloric acid. CO 3 2 HCO 3 3H 2H 2O 2CO 2. Completion of reactions a b and c.
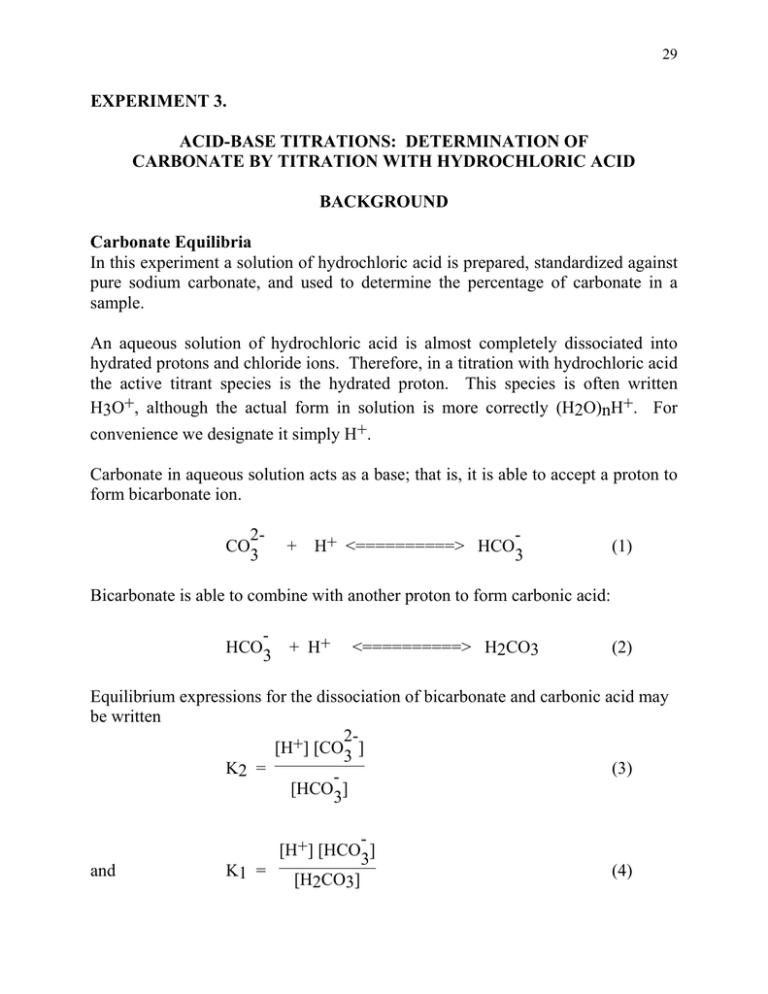
HC03- H H2CO3 C032- 2H H2CO3 A separate aliquot of unknown is treated with excess standard NaOH to convert HCO3- to CO32-. Analysis of a Mixture of Carbonate and Bicarbonate HCl 01117 M NaOH 01018. In this experiment a solution of Na2CO3 will be titrated with a solution of HCl.
In that case a back titration should be used. Equivalence point of the diprotic titration curve. Because this species can act as both an acid and a base both equilibria must be considered.
Titration of Sodium Hydroxide and Sodium Carbonate Phenolphthalein. The mixture is then further neutralised by a known concentration and volume of sodium hydroxide solution NaOH to determine the number of mole of HCl that reacted with CaCO3 in the toothpaste. Add excess H2SO4 until all signs of bubbling stops Make sure to record the volume and molarity of the H2SO4 added.
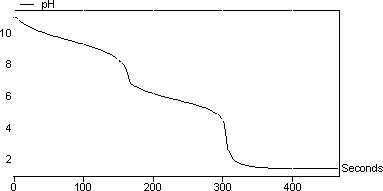
Heat the mixture to drive of the CO2 gas. The shape of the pH titration curve will be observed and the Kb values for the base will be determined. Mass unknown 22187 g.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. A standard solution of 0100 M HCl will be provided. If you cant see the image clearly please click on it.
The mixture was then titrated against 0. CO 3 2 H HCO 3. A bromcresol green-methyl red indicator is added and the titration continues to the second endpoint at a pH between 43 and 49.
Titration of Sodium Carbonate With Sodium Bicarbonate After Second End Point for Methyl Orange. The titration curves for potassium bicarbonate alone clearly exhibit only one EP for potassium bicarbonate while the titration curves for the solution withpotassium bicarbonate and potassium carbonate have two EPs. A OHˉ H ----H₂O b CO₃²ˉ H----HCO₃ˉ c HCO₃ˉ H----H₂O CO₂ H corresponds to standard HCl while hydroxide carbonate and bicarbonate are the alkalinity causing.
A back titration is also known as indirect titration. The pH of the solution will be monitored as the HCl is added with a pH probe attached to a CBL. Total amount of acid used represents the total alkalinity.
2NaHCO3 Na2CO3 CO2 H2O. Pipette out 2500mL of the given carbonate solution into a titration flask add few drops of phenolphthalein indicator and titrate the solution with 01M HCl. At this point the amphoteric6 intermediate species is the only species present.
D Bicarbonate and Hydroxide alkalinities cannot be present together e All hydroxide alkalinity is neutralized by pH 100 f all Carbonates are converted to bicarbonates by pH 83 Group Result of Hydroxide Carbonate Bicarbonate Titration Alkalinity Alkalinity Alkalinity A P 0 0 0 T initial pH 83 B P 05T 0 2P 0 C P T T 0 0. Repeat your titration using methyl orange indicator instead of phenolphthalein. When HCl solution is added slowly to the Na 2 CO 3 solution reaction happens in two stages.
When dilute HCl is slowly added first sodium bicarbonate and sodium chloride are formed. Carbonate and bicarbonate ions ie. Sodium bicarbonate is basic and can reacts with HCl furthermore.
A known mass of toothpaste is neutralised with a known concentration and volume of hydrochloric acid HCl. The first equivalence point corresponds to the added potassium carbonate while the second one corresponds to the sum of potassium. Note that each mole of carbonate requires two moles of acid for complete titration.
In the case of the carbonate CO 3 2- titration the intermediate species is hydrogen carbonate or bicarbonate HCO 3-. In one of the titrations of sodium carbonate with HCl there was one question asked. This value indicates the total T alkalinity and is a measure of all carbonate bicarbonate and hydroxide in the sample.
Problems this would not work if the mixture contains another solid that is soluble in HCl for instance salt or sugar. Volume_hcl Volume Of Sodium Carbonate2Volume Of Sodium Bicarbonate Go. Hydroxide and one-half of the carbonate in the sample.
This is ok as a primary standard. First total alkalinity moles of bicarbonate moles of carbonate is measured by titrating the mixture with standard HCI to a methyl orange end point. Volume HCl delivered after NaOH added second titration.
HC03- OH- CO32- H2O Then all the carbonate is precipitated with BaCl2.

Titration Stoichiometry Openstax Cnx

Solution Lab Report Analysis Of A Carbonate Bicarbonate Mixture Studypool
Warder Titration Simultaneous Determination Of Sodium Hydroxide And Carbonate

Solution Lab Report Analysis Of A Carbonate Bicarbonate Mixture Studypool
How To Determine The Standardisation Of Hcl Using Sodium Carbonate Quora
Science Experiment Acid Base Titrations Results

Titration Of A Mixture Of Carbonate And Bicarbonate
Experiment 3 Acid Base Titrations Determination Of

Titration Of A Mixture Of Carbonate And Bicarbonate

Chm 212 Experiment 3 Analysis Of A Mixture Of Carbonate And


Posting Komentar untuk "Carbonate Bicarbonate Titration With Hcl"