Carbonate Ion Bond Order
Carbonate Ion is a polyatomic ion with formula of CO3 2-. There are two resonating structure will be possible of HCO3 ion.
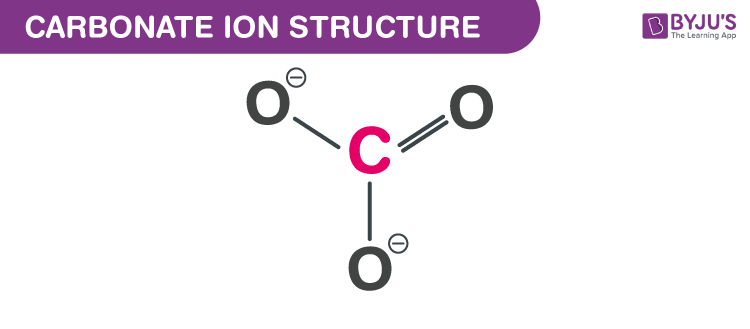
How To Calculate Bond Order Of Co32 Chemistry Q A
Therefore 4 is the bond order of CO2.
Carbonate ion bond order. If the bond order is zero the molecule cannot form. In molecules that have resonance bonding the bond order does not need to be an integer. Secondly what is the bond order of carbonate.
As an CaCO 3 can be given. The higher bond orders indicate greater stability for the new molecule. Bond order is the number of bonds between a pair of atoms.
What is the C-O bond order in the carbonate ion CO 3 2-. It is determined by adding all the outer shell electrons in the bond subtracting the non-bonding electrons and diving the result by 2. Enter a decimal number In which species CO3 or CH3CO0 are the C-O bonds longer.
There are three σ bonds and π bond around carbon atom in the Lewis structure of CO. In some compounds or ions it is impossible to fully describe the bonding in terms of one structure. CARBONATE ANION By considering its resonance delocalization the bond order on this molecular ion can be determined.
What is the C O bond order in carbonate. For polyatomic molecules bond order can be calculated by making the resonance structures. According to experimental findings all carbon to oxygen bonds in CO_32- are equivalent.
Carbonates are readily decomposed by acids. What is the bond order of the C - O bonds in the carbonate ion. Therefore carbonate ion is described as a resonance hybrid of the following structures Carbonate Ion as a Resonance Hybrid.
Consider the carbonate ion CO 3 2-carbon C has four valence electrons x 1 carbon 4 e-oxygen O has six valence electrons x 3 oxygens 18 e-The ion has an overall negative two charge so we add 2 e-to give a total of 24 e-to be placed in the Lewis structure. The resonance structures of lead carbonate ion are Total number of bonds existing in the lead carbonate ion is four. The bond length of C-O bond is 142 pm CO is 116 pm whereas the bond length of carbonate ion is 131 pm.
Solitary bonds binding in lead carbonate ion is three. Y the value of x y will be. Non-bonding electrons 8.
For CO2 Total electrons 16. Click hereto get an answer to your question If the bond order of carbonate ion CO2 - 3 is expressed by the simple ration x. Since there are 3 structures the answer is 2 1 1 3 133333 or 4 3.
In acetylene HCCH the carbon-carbon bond order is also 3 and the CH bond order is 1. It is a conjugate base of a hydrogencarbonate. So 1682 4.
The formula for calculating through resonance structure is written as. Show activity on this post. Metal carbonate compounds are common in the world.
Number the C-O ie. CO 3 2-Lewis structure. For the bond order identify the bonds in the structure.
For carbonate ion CO32 the resonating structures are given as follows. Carbonate ion CO 3 2-Carbonate ion has a -2 charge. NCI Thesaurus NCIt Carbonate is a carbon oxoanion.
Average Bond Order Formula. In which species CO3 or CH3CO0 are the C-0 bonds weaker. Since there are 3 structures the answer is 2113133333 or 43.
The bond order is 2 not 4 for ceCO2 and the bond order for the carbonate ion is somewhere between 1 and 2 due to resonance. Bond order of 1 CO is 2 and bond order of C-O is 1. For example in diatomic nitrogen NN the bond order is 3.
The bond orders of the CO32 will be 1 and 3. We should notice that for the pi bond order there will be 2 pi electrons delocalized onto three bonds making each bond on average having 23 of a pi electron thereby giving a pi bond order of 13. Each of the single bonded oxygen atoms in the carbonate ion has a bond order of 15 and a formal charge of -1 each.
This affords a bond order for the carbon - oxygen bond of 1 13. It has 3 resonating structures and total four bonds are present in one structure. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-.
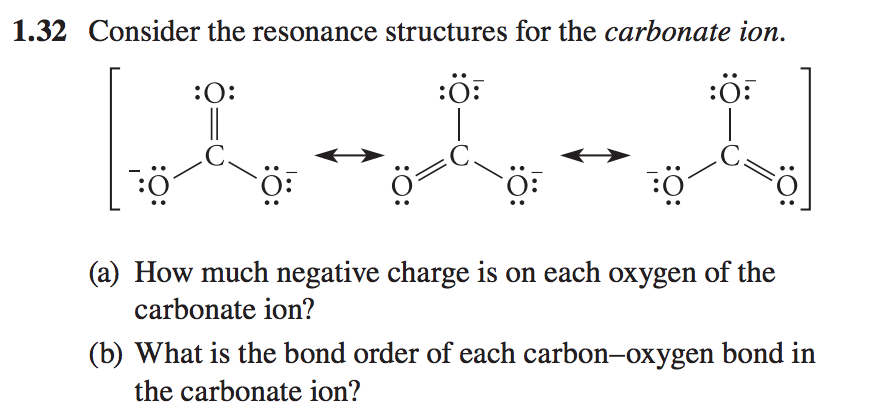
Applying this same method to the carbonate ion we have 3 resonance structures with bond orders of 2 1 1 when considering the bond between carbon and a single oxygen. Applying this same method to the carbonate ion we have 3 resonance structures with bond orders of 2 1 1 when considering the bond between carbon and a single oxygen. What is the bond order of each carbon oxygen bond in the carbonate ion In a carbonate ion there is one double bond oxygen CO and two single bonded oxygen C-O.
Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. Average Bond Order is given by rmAveragermbondrmorder fracrmNumberrmofrmbondsrmNumberrmofrmresonancermstructures. Thus the carbonate ion has the longest bond length followed by carbon dioxide and finally carbon monoxide.
What Is The Bond Order Of Co32 Quora

A Potassium Ion Has A 1 Charge It Would Form An Ionic Bond Because It Would Donate One Electron And Carry A Positive Charge Ionic Bonding Positivity Math

Important Disaccharides Mcat Molecules Math

6 Step Organic Chemistry Reaction Identification Functional Group Organic Chemistry Reactions Problem Solving
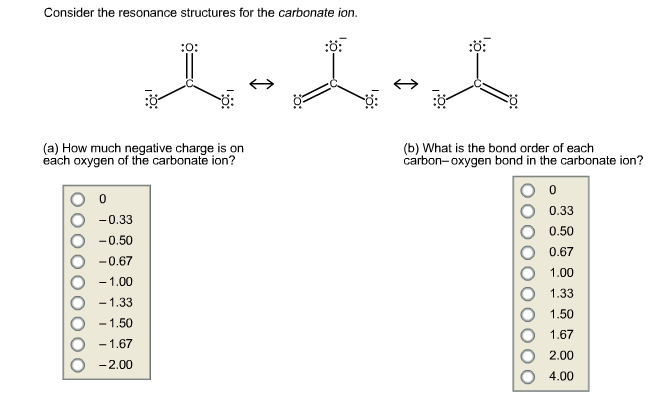
Solved Consider The Resonance Structures For The Carbonate Chegg Com

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Simple Method For Writing Lewis Structures Ozone O3 And Carbonate Co3 2 Molecular Geometry Writing Chemistry

How To Calculate Bond Order Of Co32 Chemistry Q A

Valence Bond Structure Of Carbonate Ion Co3 2 Download Scientific Diagram

Inorganic Chemistry Laminated Study Guide 9781423214311 Chemistry Study Guide Chemistry Lessons Chemistry Education

Solved 1 32 Consider The Resonance Structures For The Chegg Com

Structural Isomer Easy Science Science Facts Science Memes Science Rules

Water Chemistry Chemistry Study Chemistry Organic Chemistry
Posting Komentar untuk "Carbonate Ion Bond Order"