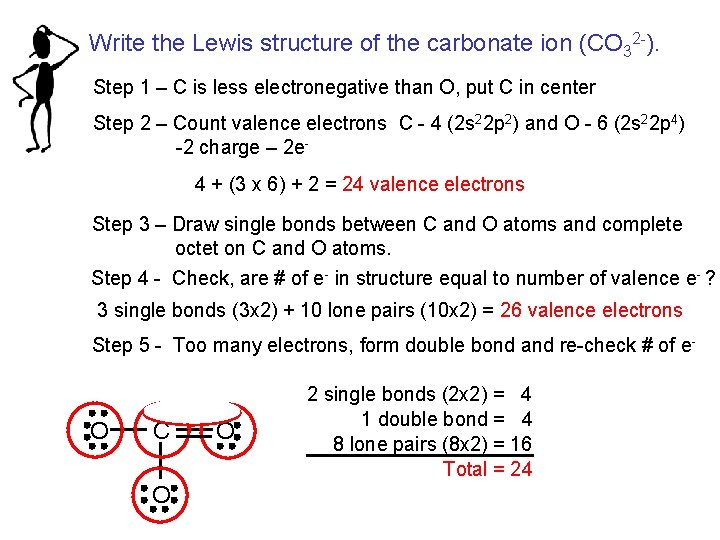
Carbonate Valence Electrons
First we need to count the total number of valence electrons. 4 plus 18 plus 2.

Draw A Complete Lewis Structure For The Carbonate Ion Co32 Include All Valence Lone Pairs In Your Answer Study Com
Carbon has 4 valence electrons each oxygen has 6 valence electrons and there are 2 more for the 2 charge.
Carbonate valence electrons. First we need to count the total number of valence electrons. Add that all up. When we see an H in front of a polyatomic structure like the CO3- here that means the H will be attached on the outside of one of the Oxygens.
So carbon has four electrons in its valence shellOxygen is located at 6 th group. We are given the following molecule. This case it is negative 2 so we add two electrons.
Be and B dont need 8 valence electrons. It has a molecular mass of 6001 gmol and carries a total formal. O 6 x 3.
O 6 x 3. Likewise what is a carbonate ion. Carbon and oxygen together have a total of 10 electrons in the valence shell.
The carbonate ion is the simplest oxocarbon anionIt consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D 3h molecular symmetryIt has a molecular mass of 6001 gmol and carries a total formal charge of 2. It consists of one carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with D 3h molecular symmetry. Generally Oxygen atom shows -2 oxidation state in a compound.
Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency or valence of an atom. Carbon is a group IVA element in the periodic table and has four electrons in its last shell valence shell. This gives 4 3 6 2 24 valence electrons.
Carbon is the least electronegative put that at the center. Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property. Carbon is located at group 4 in the periodic table.
When dissolved in water sodium carbonate forms carbonic acid and sodium hydroxide. 4 electrons - 2 electrons from 2p orbital and 2 from 2s orbital. The Carbonate CO23 Ion Carbon has 4 valence electrons each oxygen has 6 valence electrons and there are 2 more for the 2 charge.
Total valence electrons given by oxygen atoms 6 3 18. Total electrons around the valence shells in the carbonate ion 24. Valence electrons of C 4 C 4.
It has six electrons in valence shell. How many valence electrons does it have. The sum of valence electrons from the constituent atoms 4 from carbon 3 x 6 from oxygen 22.
Cobalt is also in Group 9 so it must have 9 valence electrons. The carbonate ion is the simplest oxocarbon anion. Thus Carbon can show max.
In eqrm CO_32- eq C is the least electronegative atom. As a strong base sodium hydroxide neutralizes gastric acid thereby acting as an antacid. See full answer below.
Also Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. Lewis dot structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
Oxygen has six we have 3 Oxygens and this negative 2 means we have an extra two valence electrons. Carbonates are readily decomposed by acids. Valence electrons of O 6 O 6.
CO32- is called a carbonate ion. NCI Thesaurus NCIt Carbonate is a carbon oxoanion. Hence in the Lewis structure the C atom is the central atom.
Subsequently question is how many electrons are present in a Co 3 ion. It is a conjugate base of a hydrogencarbonate. Thus the ion has gained two electrons with respect to its constituent atoms therefore it must carry a.
For the CO32- Lewis structure there are a total of 24 valence electrons available. Each hydrogen atom group 1 has one valence electron carbon group 14 has 4 valence electrons and oxygen group 16 has 6 valence electrons for a total of 21 4 6 12 valence electrons. Question 20 of 25 40 40 Points Draw the Lewis structure for the carbonate polyatomic ion.
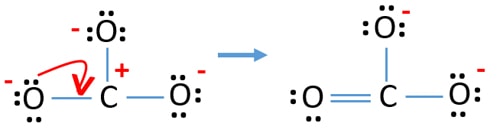
Complete info about it can be read here. E 2 --- we add electrons when the charge of the ion is negative in. Since four of the shared electrons come from the oxygen atom and only two from.
The valencies of the elements belonging to the s-block and the p-block of the periodic table are generally calculated as the number of valence electron or eight minus the number of valence electrons. Carbon can release at max. We just add the valence electrons of each atoms involved.
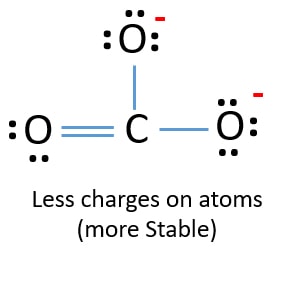
Following the octet rule for both carbon and oxygen the two atoms form a triple bond with six shared electrons in three bonding molecular orbitals rather than the usual double bond found in organic carbonyl compounds. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. This gives 4 3 6 2 24 valence electrons.
There are three oxygen atoms in CO 3 2-ion Therefore. Carbon has 4 valence electrons. We just add the valence electrons of each atoms involved.
First we need to count the total number of valence electrons. How many valence electrons does hco3. CO2 3 C O 3 2.
Total valence electrons given by carbon atom 4. CO32- is called a carbonate ion. For HCO3- we have a total of 24 valence electrons.
The number of valence electrons in C and O are 4 and 6 respectively. Now we know how many electrons are there in.

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Bonding General Concepts Chapter 8 Valence Electrons Are The Outer Shell Electrons Of An Atom The Valence Electrons Are The Electrons That Participate Ppt Download
What Is The Lewis Structure Of Co3 2 Quora

Valence Bond Structure Of Carbonate Ion Co3 2 Download Scientific Diagram

Chemical Bonding I Basic Concepts Chapter 8 Valence

What Is Carbonate S Lewis Structure Study Com

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
Lewis Structure For Co32 Carbonate Ion
Solved How Many Valence Electrons Are In The Carbonate Ion Chegg Com
Lewis Structure For Co32 Carbonate Ion
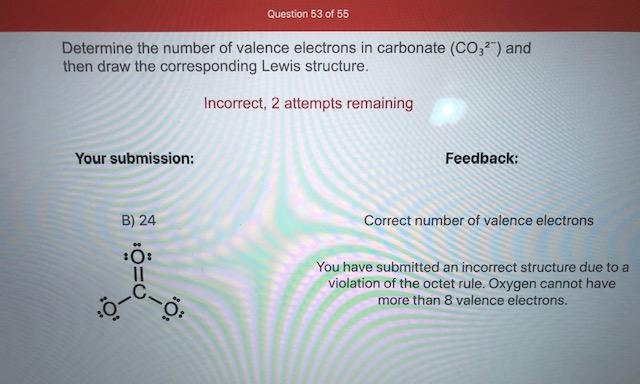
Answered Determine The Number Of Valence Bartleby

Calcium Carbonate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic
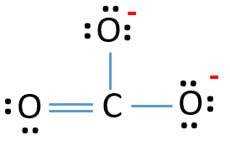
Lewis Structure For Co32 Carbonate Ion
Posting Komentar untuk "Carbonate Valence Electrons"