Calcium Carbonate G/mol
The molecular weight of calcium carbonate is 100 gmol while that of hydrochloric acid is 3645. We add a known amount of HCl equal to 2x y moles to x moles of CaCO3.

Ddt Full Form What Was Ddt Used For Is Ddt Harmful To Humans Physical Chemistry Chemistry Chemistry Notes
Molecular weight of Calcium Carbonate or grams The molecular formula for Calcium Carbonate is CaCO3.
Calcium carbonate g/mol. The Ca2 ion can be expressed in box notation. It is also found in many forms such as marble limestone etc. According to the reaction 1 mole of calcium.
Then 0001 moles of CO2 are obtained from 0001 moles of CaCO3. NHCl M ass of HCl M olar mass 110g 36458 gmol 0302mol n H C l M a s s o f H C l M o l a r m a s s 110 g 36458 g m o l 0302 m o l. Calcium Carbonate is available widely ad a natural inorganic compound and other names for the chemical compound are chalk limestone or marble etc.
The SI base unit for amount of substance. It can also be used as a pigment and filter making possible the production of a whiter and higher quality pigment compared to other minerals. We will see that how many moles are there in 24cm3 of CO2.
From the formula of calcium carbonate 1 mole of CaCO3 C a C O 3 contains 3 mole oxygen. The answer is 00099913175450533. Therefore 1 g of calcium carbonate has 6021023100 602 1021 molecules.
Answer The fictional ppm calcium carbonate in the sample can be determined by findingmg L of CaCO 3. The molecular formula for Calcium Carbonate is CaCO3. How many moles Calcium Carbonate in 1 grams.
Calcium carbonate is employed largely in the paper and pulp industries. 2x moles of HCl react with the CaCO3 leaving y moles of unreacted HCl in the flask. Mw M w is the molecular weight.
What is Calcium Carbonate. Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms where the use of chalk a form of CaCO3 is found. It has a melting point of 1339oC and reacts readily with acids making it a base.
Molecular weight of Calcium Carbonate or mol. You can convert this into grams using the MW we calculated in part 2 for calcium carbonate and the following equation. Calcium carbonate decomposes when heated to give calcium oxide and carbon dioxide.
Molar mass of calcium carbonate is 100 g mol which means 100 g calcium carbonate has 6021023 molecules Avogadro number. Convert grams Calcium Carbonate to moles or moles Calcium Carbonate to grams. Calcium Carbonate is an odorless and tasteless powder or hexagonal crystal.
It is made up of a calcium ion Ca2 and a carbonate ion CO 3 2-. Therefore 183 g of calcium chloride will be produced by the reaction of 270 g of calcium carbonate with 120 g of hydrochloric acid. Molar mass of CaCO3 1000869 gmol.
CaCO3 s 2HClaq CaCl2 aq H2Ol CO2 g Next draw a figure showing relative amounts of the reactants. Begin as above then use water displacement to measure the volume of carbon dioxide produced. It is not realistically possible to measure the enthalpy change of this reaction directly but using the fact that both calcium carbonate and calcium oxide react directly with hydrochloric acid a Hess law cycle can be.
It is found in the earths crust. Molesvolumemolar gas volie fixed at 24dm3 or 24000cm3 thus if 1 mole of Calcium Carbonate gives 1 mole of Carbon dioxide. The molecular mass of calcium carbonate 1000869 gmol.
The SI base unit for amount of substance is the mole. Again a mole ratio will be necessary to calculate the amount of calcium. Calcium carbonate is used therapeutically as a phosphate buffer in hemodialysis as an antacid in gastric hyperacidity for temporary relief of indigestion and heartburn and as a calcium supplement for preventing and treating osteoporosis.
It has a molar mass of 1001 gmol. CaCO 3 EDTA 4 aq à CaEDTA2 aq CO 3 2 aq. We titrate the excess HCl with y moles of NaOH.
Calcium ii carbonate 11 marble. 40078 120107 1599943 Percent composition by element. Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3.
Application of Calcium Carbonate. The calcium chloride can be used as the calcium carbonate is above although a mole ratio is required. CaCO 3 s CaOs CO 2 g.
Calcium Carbonate is the carbonic salt of calcium CaCO3. The balanced equation of the reaction is taken to be. Mass g No.
The decomposition of calcium carbonate. We assume you are converting between moles Calcium Carbonate and gram. This method takes the longest.
The chemical formula for the compound is CaCO3 and its molecular weight is approximately 1001 gmol. Aragonite cianciano sicily carbonic acid calcium salt. Calcium Carbonate molecular weight.
CaCO 3 1001 g mol State the value to the nearest ppm. Calcite with silicate constituent. Calcium carbonate 85 concentrate.
Calcium carbonate has a molecular weight of 1000869 gmol. The equation above depicts that 100 g of calcium carbonate can be dissolved in 729 g of hydrochloric acid. Moles in 24cm3 of CO2.
Carbon C Calcium Ca Oxygen O Molecular weight. Determine the total hardness in mg L ppm of calcium carbonate. 1 grams Calcium Carbonate is equal to 00099913175450533 mole.
Calcium Carbonate CaCO 3 is a mineral white in colour and is the main component of different shells. Now it is given that 24cm3 of CO2 is obtained. Moles x MW Mass g 0005 mol x 100059 gmol-1 05 g 5 Determine Actual Yield and Percentage Yield Your actual yield is the amount of product in g that you actually produced.
You can view more details on each measurement unit.

How Many Moles Of Calcium Carbonate Caco 3 Are Present In 10 G Of The Substance Youtube

Calcium Carbonate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

Aqa Gcse Chemistry Paper 1 Quantitative Chemistry Complete Revision Summary Notes Video Q A Gcse Chemistry Chemistry Paper Gcse Chemistry Revision
What Is The Number Of Molecules Present In 10 G Of Caco3 Quora

Pin By Barbara On Kurt Cobain Crude Writing Word Search Puzzle

Question Video Calculating The Mass Of Calcium Carbonate Required To Produce A Given Mass Of Calcium Oxide Nagwa

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Atom Diagram Electron Configuration

How Many Moles Are 5 Grams Of Calcium Atomic Mass Of Calcium 40 U Youtube

Molar Mass Molecular Weight Of Caco3 Calcium Carbonate Youtube

Buy Limestone Powder In 2020 Limestone Powder Stuff To Buy
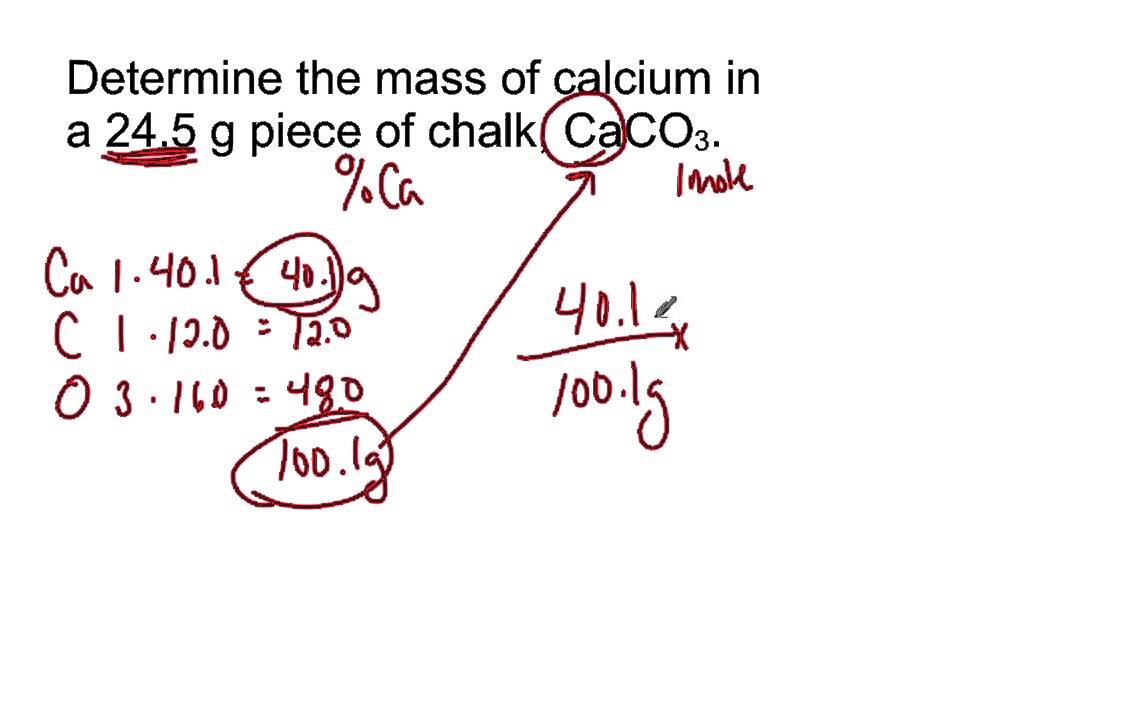
Mass Of Ca In 24 5 G Sample Of Calcium Carbonate Youtube
Calculate The Molecular Mass Of Caco3 At Mass Ca 40 U C 12 U O 16 U Cbse Class 9 Learn Cbse Forum
Calcium Carbonate Caco3 Pubchem

Pin On Sat Chemistry Subject Tests
Posting Komentar untuk "Calcium Carbonate G/mol"