Carbonate Alkalinity Equation
The multiplier 01 then converts this to total alkalinity as mgL CaCO 3. CA nearly equals TA Calculations Any two of the four CO 2 properties ΣCO 2 P CO2 pH and carbonate alkalinity can be used to determine the CO 2 system Traditionally pH and alkalinity were measured.
Apparently some chemists decided this topic was not confusing enough as it is.
Carbonate alkalinity equation. Carbonate CO 3 2- Alkalinity as CaCO 3 Bicarbonate HCO 3- Alkalinity as CaCO 3 p alk 0 0 0 t alk p alk ½ t alk 0 2p alk t al 2p alk p alk ½ t alk 0 2p alk 0 p alk ½ t alk 2p alk t alk 2t alk p alk 0 p alk t alk t alk 0 0 MANTECHs MT Series automates alkalinity and low alkalinity measurements following EPA ASTM ISO and Standard Methods. Yes you read that right. CaCO 3 H 2O CO 2 Æ CaHCO 3 2 CaCO 3 has a molecular weight of 100 gmol The HCO 3-anion has a molecular weight of 61 gmol Therefore each mol of CaHCO 3.
We then review different methods that have been used to solve the total alkalinitypH equation with a main focus on bio-geochemical models. Note that we are ignoring contributions of PO4 and SiO4 to alkalinity which may be important regionally but probably lead to errors in pCO2 1 ppmv globally See. CaCO 3-CO 2-H 2 O system Calcium carbonate in water with a fixed partial pressure of carbon dioxide.
We dont want to get too nerdy. As we assumed all carbonate came from calcium carbonate we can write. To solve it we need at least one more independent equation to match the number of unknows.
Carbonate Alkalinity CaCO 3 mgL 100000 K 2 HCO 3-10 pH And if you really want to convert carbonate alkalinity from units of CaCO 3 to units of CO 3 2- this source shows you how to do so. Alkalinity is a water characteristic that quantifies the capacity of water to neutralize acids namely accepts hydrogen ions H. 2 x 170 340.
This procedure uses an equation derived from the slope of the line described above to extrapolate back to the amount of sulfuric acid that was added to actually convert all the bases to carbonic acid. Carbonate equilibrium system based on P. Point and is the endpoint of the caustic alkalinity and total acidity titrations.
The following steps outline the procedures necessary to determine the alkalinity of your sample. Total alkalinity can be calculated using two different equations Equations 6 and 7. AC HCO3 2CO 3 2 b 2c 915 If the water contains Ca2 or Mg2 and carbonate or is in contact with calcite also the dissociation equilibrium of calcite affects the carbon chemistry.
Section 5- Carbonate Chemistry 22 AlkalinityOHHCO 3 2CO 3 OHHCO 332CO 18 Therefore there is no alkalinity in a CO 2-water system unless other sources of base are added. 250 170 80. CA nearly equals TA.
The solution should contain significant concentration of bases stronger than carbonates. 15 10 15 ppm CaCO3. As a strong base sodium hydroxide neutralizes gastric acid thereby acting as an antacid.
340 250 90 mgL hydroxide alkalinity Carbonate alkalinity. ML of acid 10 ppmmL For example if 15 mL of acid were used in the titration then the total alkalinity would be. Cs ceCaCO3 ceH2CO3 HCO3- CO32-.
The equations above are restricted to carbonate systems without other weak acids or bases. The phenolphthalein alkalinity of 170 mgL is more than one-half of the total alkalinity so use Row 5. This is the endpoint for carbonate alkalinity and CO 2 acidity titrations.
When a waters pH is above 83 its alkalinity tends to come from carbonate ions and below that threshold the alkalinity usually comes from. Ed Environmental Chemistry 1996. Tion based upon carbonate-borate-alkalinity is presented.
Procedure to calculate carbonate and bicarbonate alkalinity Titration from pH 83 to 45 measures the remaining one half of the carbonate bicarbonate. Anyway heres the formula. Note that this simple formula requires that you use the exact acid concentration and sample volume listed in this procedure.
80 x 2 160 mgL carbonate alkalinity Bicarbonate alkalinity. Alkalinity is usually measured in milligrams per liter of calcium carbonate which is a calcium ion bound to a carbonate ion. 2 At pH 83 the H 2CO 3 equals the CO 3 2-.
Amount of acid required to reduce the pH of an alkaline solution to 108. We then present two variants of a new robust and universally convergent al-gorithm to solve the. By an acid titration referred to as the total alkalinity thus about equals the carbonate alkalinity defined as.
When dissolved in water sodium carbonate forms carbonic acid and sodium hydroxide. Carbonate Alkalinity CA CA 2CO 3-2 HCO 3- Typically HCO 3-and CO 3-2 are present at 1000x conc of other proton acceptors Hence. Carbonate alkalinity is especially important in environmental contexts.
Carbonate Alkalinity as CO 3 2-mgL 06 Carbonate Alkalinity as CaCO 3 mgL Converting Bicarbonate Alkalinity from mgL as CaCO 3 to mgL as HCO 3-Consider the following reaction. Alkalinity mg CaCo 3 L A N 50000 Hardness and. Once M alkalinity and P alkalinity is known we are able to calculate the total amount of dissolved inorganic carbon.
Alkalinity d Bicarbonate and Hydroxide alkalinities cannot be present together e All hydroxide alkalinity is neutralized by pH 100 f all Carbonates are converted to bicarbonates by pH 83 Group Result of Hydroxide Carbonate Bicarbonate Titration Alkalinity Alkalinity Alkalinity A P 0 0 0 T initial pH 83 B P 05T 0 2P 0 C P T T 0 0. Carbonate Alkalinity CA CA 2CO 3-2 HCO 3- Typically HCO 3-and CO 3-2 are present at 1000x conc of other proton acceptors Hence. Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property.
3 M P H 2 CO 3 HCO 3- CO 3-2 DIC. The shortcomings and limitations of these methods are made out and discussed. In the alkalinity titration virtually all of the CO 3 2-has reacted thus the term carbonate alkalinity and half of the HCO 3.
Tans Why Carbon Dioxide from Fossil Fuel Burning Wont Go Away In. What we need is the equation for the material balance of the system. Alkalinity of natural water is mainly due to the presence of two forms of the carbonate ions denoted as HCO3- and CO32- that act as a buffer system.
Table 2 shows the equation endpoints of carbonate solutions explaining the acidity and alkalinity definitions described above.

The Geochemistry Of Natural Waters The Carbonate System
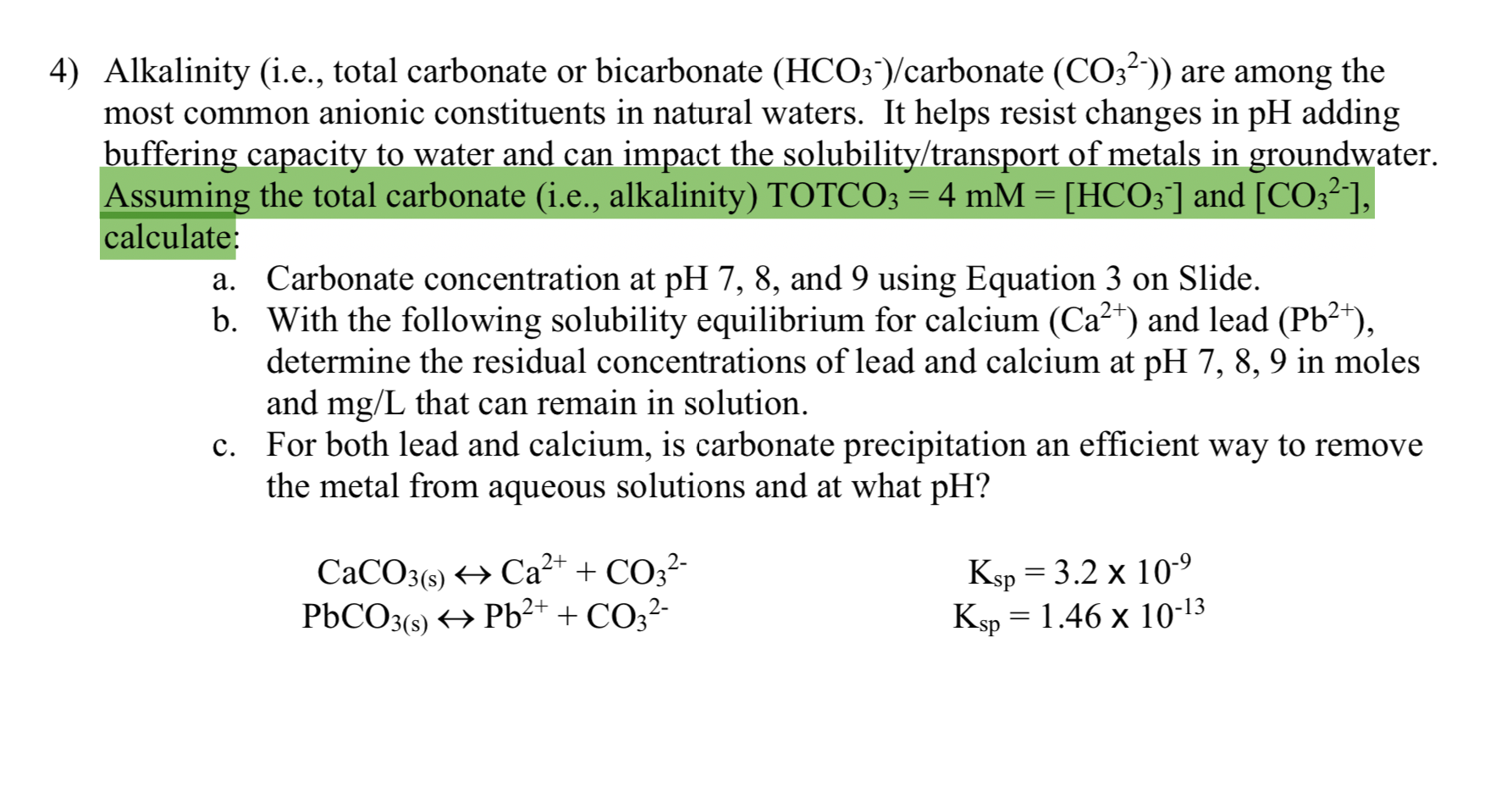
4 Alkalinity I E Total Carbonate Or Bicarbonate Chegg Com

Ppt Carbonate System Alkalinity Powerpoint Presentation Free Download Id 1837976

Carbonate Alkalinity Vs Corrected Alkalinity

Is There Corelation Between Ph Alkalinity And Bicarbonate In Water
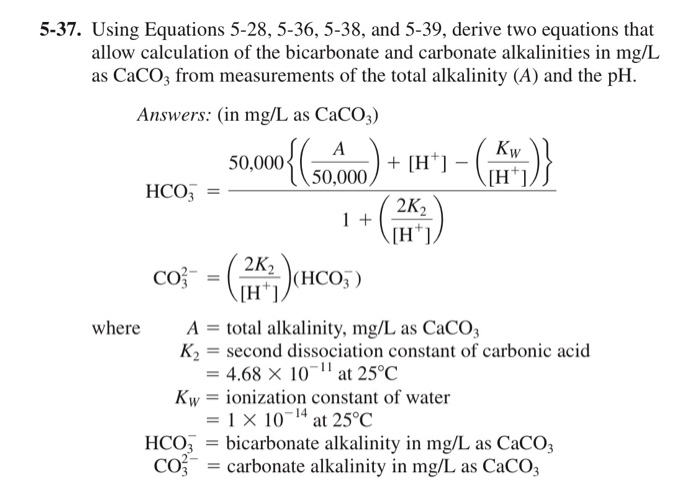
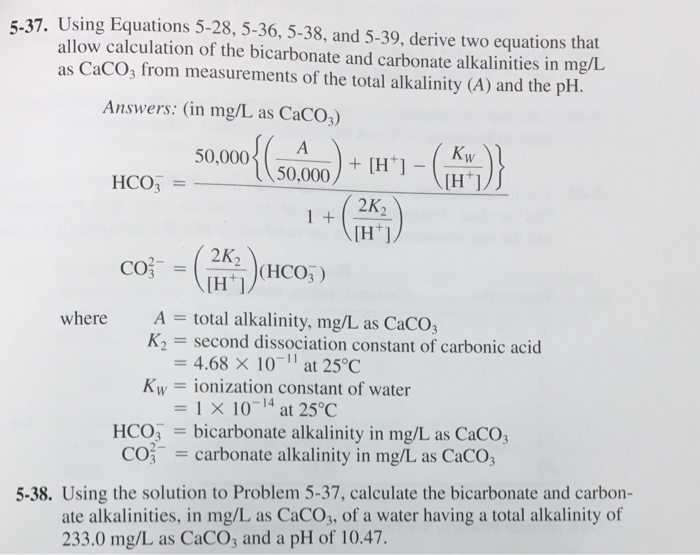
Solved 5 37 Using Equations 5 28 5 36 5 38 And 5 39 Chegg Com
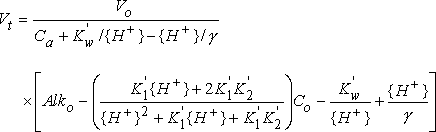
Methods For Alkalinity Calculator
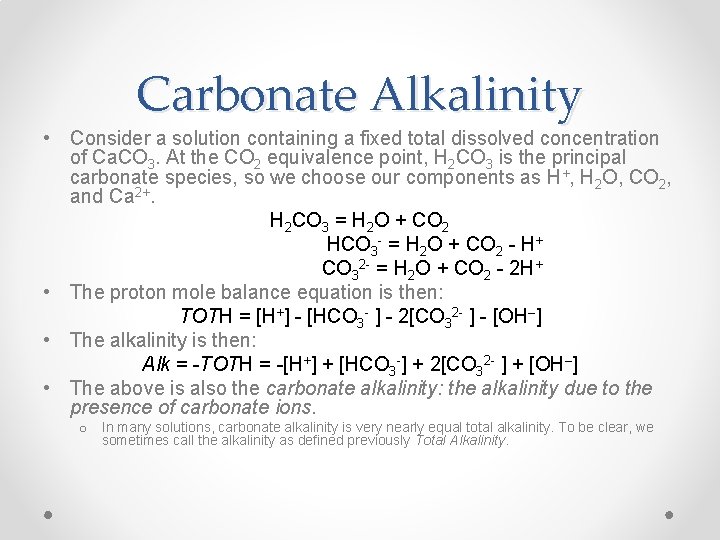
Carbonate System Alkalinity Lecture 21 Toth Toth Is

Solved 5 37 Using Equations 5 28 5 36 5 38 And 5 39 Chegg Com

Carbonate System Alkalinity Lecture 21 Toth Toth Is The Total Amount Of Component H Rather Than The Total Of The Species H O Every Species Containing Ppt Download
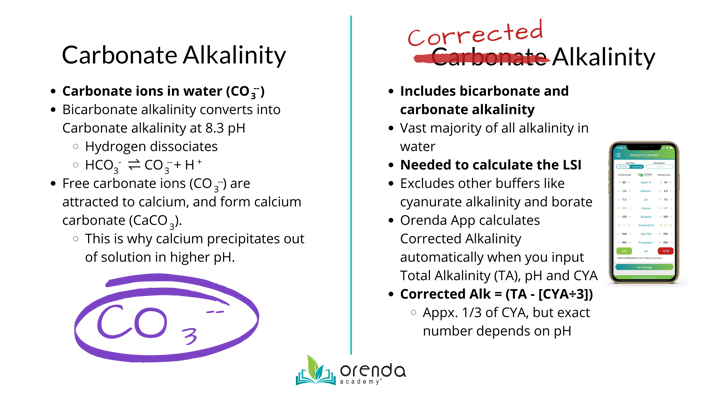
Carbonate Alkalinity Vs Corrected Alkalinity

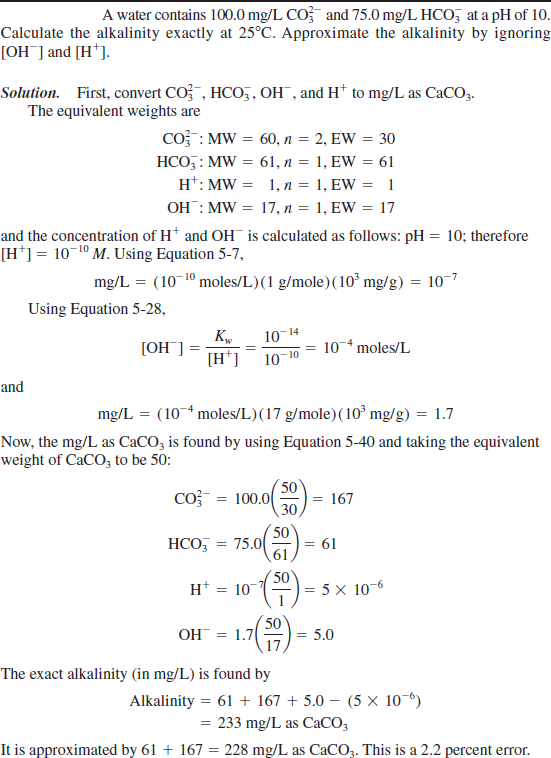

Posting Komentar untuk "Carbonate Alkalinity Equation"