Carbonate Anion Charge
Consider the following equivalent resonance structures for the carbonate anion. Confirmatory anion testing is carried out using water extract when salt is water-soluble and using sodium carbonate extract when salt is water-insoluble.

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of 2.
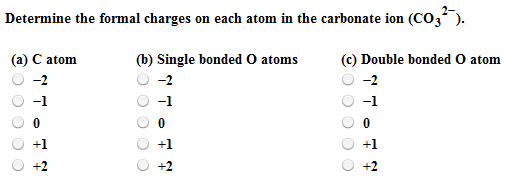
Carbonate anion charge. Carbonate is a polyatomic ionIt has -2 chargeIn carbonatewe get one carbon and three oxygens. As you know ionic compounds must be electrically neutral meaning that the overall positive charge coming from the cation must be balanced by the overall negative charge coming from the anion. Now lithium is located in group 1 of the Periodic Table which means that it forms 1 cations by giving up its sole valence electron.
What is the average charge on each oxygen atom. Dolomite is an carbonate mineral composed of calcium magnesium carbonate CaMg CO₃₂. The -2 charge on carbonate requires that iron have 2 oxidation number Guideline 2.
Calcium carbonate is an ionic compound with electrostatic interactions between the dispositive calcium cation and the dinegative carbonate anion. You will learn about these facts in. It presents a large capacity to absorb moisture.
It can be made as the product of potassium hydroxide s absorbent reaction with carbon dioxide. Salts or ions of the theoretical carbonic acid containing the radical CO2 3-. FeC03 This ionic compound contains the carbonate polyatomic anion C O3.
Carbonates are readily decomposed by acids. In this case you need two ammonium cations to balance the 2- charge of the carbonate anion. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as.
Lewis structure of carbonate ion is drawn in this tutorial step by step. Salts are hygroscopic or tend to pick up water. NCI Thesaurus NCIt Carbonate is a carbon oxoanion.
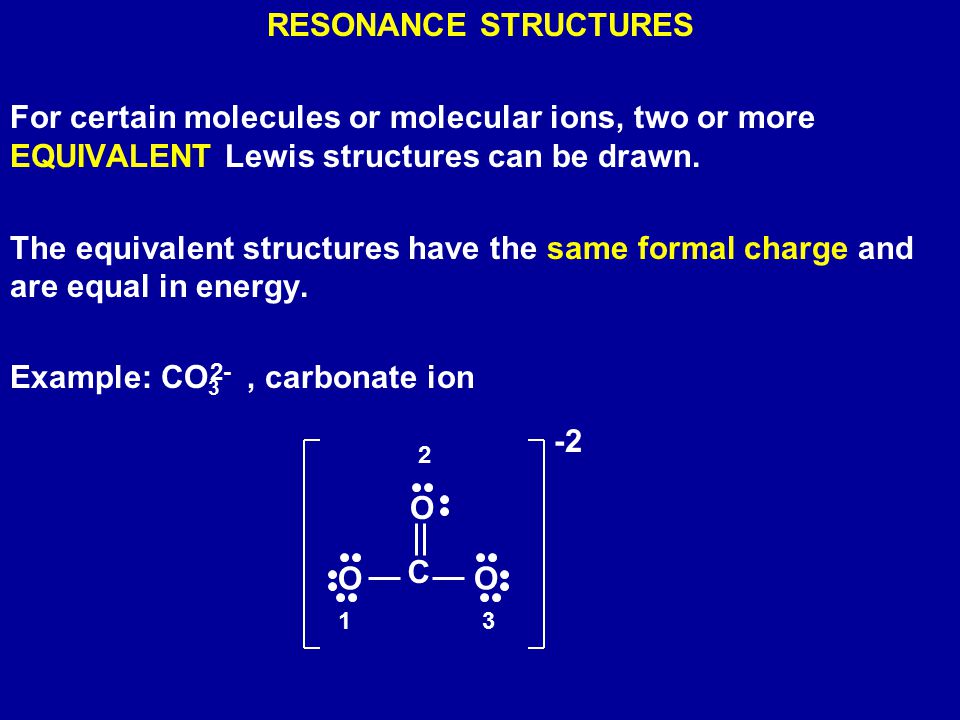
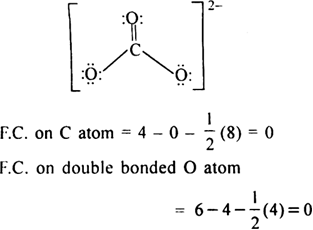
The single bonded oxygen atoms have a net charge of 1 each as they have gained a lone pair of electrons. Carbonate anion is CO₃². Oxygen ions have a 2- charge so 3 of them would equal 6- charge.
In the carbonate ion the carbon atom is bonded with a double bond to an oxygen atom and with single bonds to two oxygen atoms. The oxidation numbers must add up to the. Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solution.
Confirmation of CO 3 2 is done using aqueous salt solution or using solid salt as such as carbonate ions are contained in the sodium carbonate extract. 30 0 o А -013 В -067 O D 013 E 067 Unanswered 2 attempts left Sube. Also called a network solid where atoms are bonded by covalent bonds in a.
Bicarbonate anion hydrogencarbonate 71-52-3. Total valence electrons concept is used to draw the lewis structure of CO 32-. Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate CaCO₃.
For example table salt or sodium chloride consists of the Na cation bonded to the Cl-anion to form NaCl. After finishing the lewis structure of CO 32- there should be a -2 charge and it should be stabile structure. The carbonate anion has covalent bonds between carbon and oxygen atoms.
Chemistry questions and answers. The carbonate ion CO3 2- has an overall charge of -2. It is a conjugate base of a hydrogencarbonate.
The resulting compound carries a neutral electrical charge. Charges have not been shown. In this case you know that the anion carries a 2- charge so you can say that 1 formula unit of lithium carbonate will contain as many lithium cations as needed to get an overall 2 charge.
Structure properties spectra suppliers and links for. How is CO3 formed. The formula of the carbonate ion is CO 3 2.
Now notice that the anion carries a 2- charge. Here you can see that you can draw the ion like this with one of the oxygen atoms having a double bond with the carbon and the other two having a single bond. The nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding.
So the formula for carbonate is CO₃ 2- Formula for Carbonate. The formation of CO 3 is inferred but it appears to decay spontaneously by the route 2CO 3 2CO 2 O 2 with a lifetime much shorter than 1 minute. When electrons are transferred and ions form ionic bonds result.
This means that the double bonded oxygen atom has no charge. The ion has two units of negative charge as there are two more electrons 32 than protons 30. Carbonate Ion is a polyatomic ion with formula of CO3 2-.
C4 a carbide ion. Carbon will form an anion with a charge of 4. Now how is this so.
Carbonate ion CO32- is a polyatomic anion with 1 carbon atom and 3 oxygen atoms. By convention the cation name and formula are listed before the anion name and formula. Furthermore What is the charge on a carbonate ion The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of 2.
In the carbonate anion each oxygen atom is -2 for a total of-6. The answer to your question. Is CaCO3 a covalent network solid.
Non-silicate mineral is mineral that does not hold the silica tetrahedron. The carbonate anion is a covalent compound anion with a net charge of -2. This water is called water of hydration.
Formal Charge Problems 3 Carbonate Co3 Youtube
What Is The Lewis Structure Of Co3 2 Quora

How Is The Charge Of Covalently Bonded Atoms Determined Chemistry Stack Exchange

Berkas Carbonate Ion Delocalised Partial Charges 2d Png Wikipedia Bahasa Indonesia Ensiklopedia Bebas

Formal Charge Problems 3 Carbonate Co3 Youtube

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

In The Carbonate Anion Which Atoms Gain The Two Electrons Chemistry Stack Exchange

Valence Bond Structure Of Carbonate Ion Co3 2 Download Scientific Diagram
Example Co3 Carbonate Ion Ppt Download
Calculate The Formalcharge On Atoms In Carbonate Ion From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
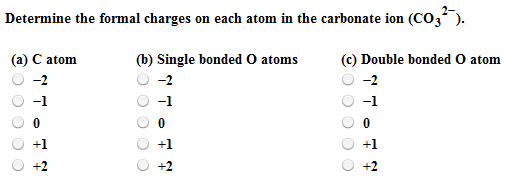
Solved Determine The Formal Charges On Each Atom In The Chegg Com

What Is The Formula Of The Carbonate Ion Clutch Prep
How To Calculate The Formal Charges For Co3 2 Carbonate Ion Youtube
Calculating Co32 Formal Charges Calculating Formal Charges For The Carbonate Ion Youtube
Posting Komentar untuk "Carbonate Anion Charge"